Uses
- For the temporary relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
- May be used as a protectant against further irritation.
Warnings
-
For use in the eyes only.
- To avoid contamination, do not touch tip of container to any surface. Do notreuse. Once opened, discard.
-
Do not touch unit-dose tip to eye.
- If solution changes color or becomes cloudy, do not use.
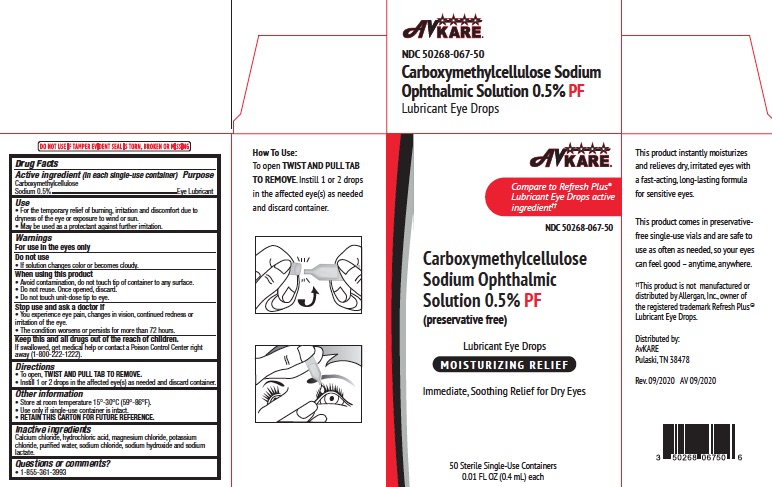
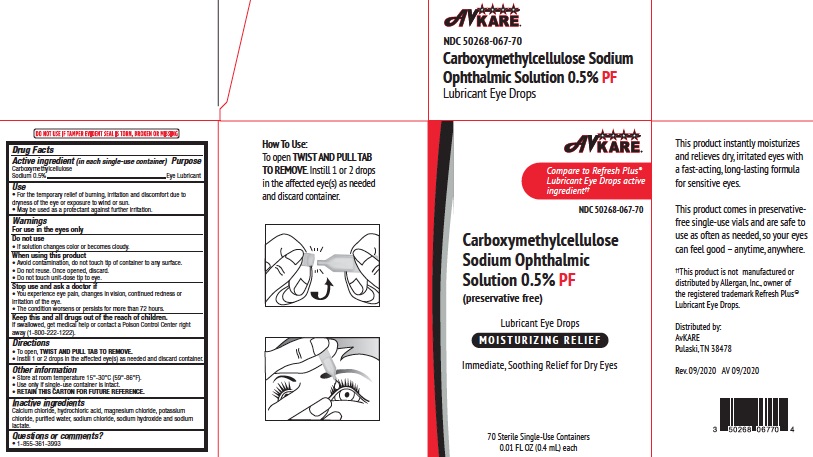
Directions
To open, TWIST AND PULL TAB TO REMOVE. Instill 1 or 2 drops in the affected eye(s) as needed and discard container.
Other information
- Use only if single-use container is intact.
- Store at room temperature 15°-30°C (59°-86°F).
- RETAIN THIS CARTON FOR FUTURE REFERENCE.