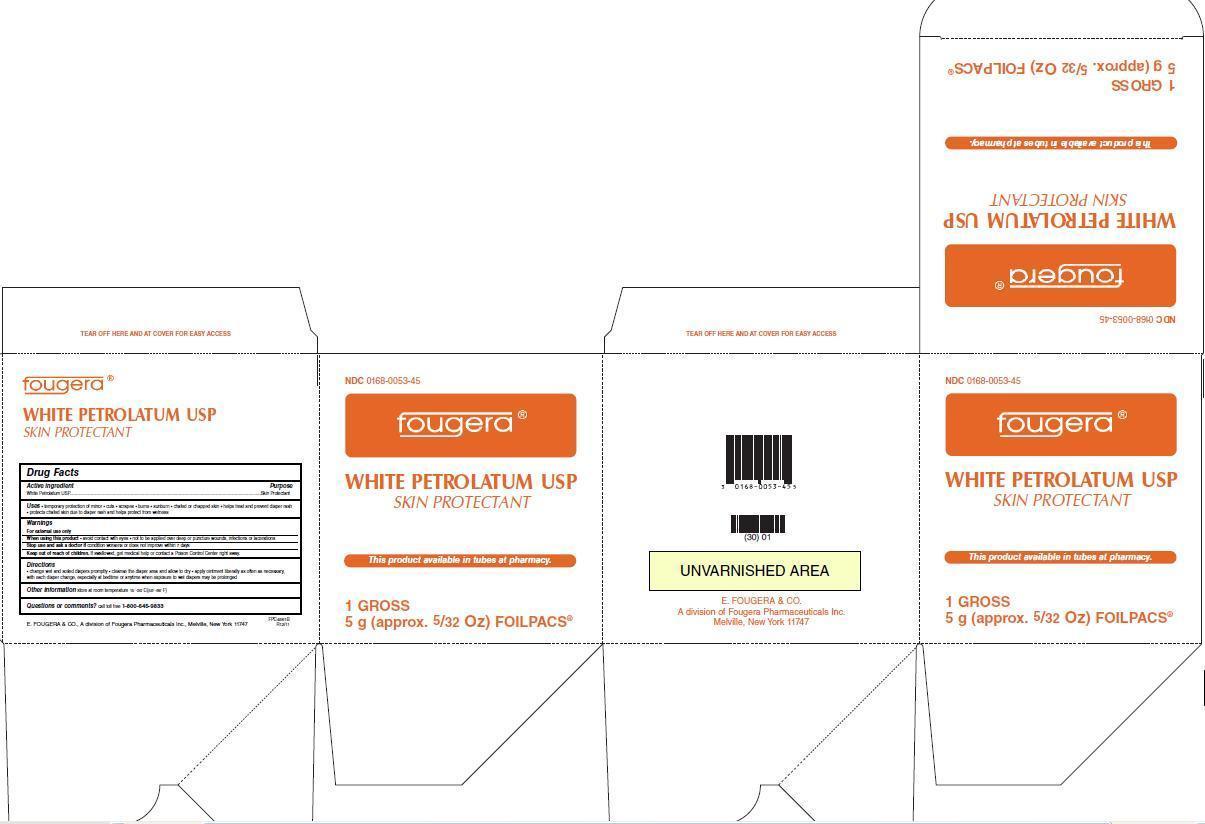
Uses:
- temporary protection of minor
- cuts
- scrapes
- burns
- sunburn
- chafed or chapped skin
- helps treat and prevent diaper rash
- protects chafed skin due to diaper rash and helps protect from wetness
Warnings:
For external use only
When using this product
- avoid contact with eyes
- not to be applied over deep or puncture wounds, infections or lacerations
Stop use and ask a doctor if
- the condition persists or gets worse
- if a rash or other allergic reaction develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions:
- change wet and soiled diapers promptly
- cleanse the diaper area and allow to dry
- apply ointment liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged
Other information:
- store at room temperature 15°-30°C (59°-86°F)
- do not sure if seal is broken or missing
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – CONTAINER
Fougera®
NDC 0168-0053-16
White Petrolatum USP
Skin Protectant
NET WT 453.6g (1 lb.)
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – CONTAINER
Fougera®
NDC 0168-0053-21
White Petrolatum USP
Skin Protectant
NET WT 28.35g (1 Oz)
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – CARTON
Fougera®
NDC 0168-0053-21
White
Petrolatum USP
Skin Protectant
Soothes minor skin irritations due to:
minor burns - scrapes - abrasions