ARFORMOTEROL TARTRATE- arformoterol tartrate solution
GLENMARK PHARMACEUTICALS INC., USA
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ARFORMOTEROL TARTRATE INHALATION SOLUTION safely and effectively. See full prescribing information for ARFORMOTEROL TARTRATE INHALATION SOLUTION.
ARFORMOTEROL TARTRATE Inhalation Solution Initial U.S. Approval: 2006 INDICATIONS AND USAGEArformoterol tartrate inhalation solution is a long-acting beta2-adrenergic agonist (beta2-agonist) indicated for:
Important limitations of use: DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHSInhalation solution (unit-dose vial for nebulization): 15 mcg/2 mL solution (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling. Revised: 12/2020 |
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Maintenance Treatment of COPD
Arformoterol tartrate inhalation solution is indicated for the long-term, twice daily (morning and evening) maintenance treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. Arformoterol tartrate inhalation solution is for use by nebulization only.
1.2 Important Limitations of Use
Arformoterol tartrate inhalation solution is not indicated to treat acute deteriorations of chronic obstructive pulmonary disease [see Warnings and Precautions (5.2)].
Arformoterol tartrate inhalation solution is not indicated to treat asthma. The safety and effectiveness of arformoterol tartrate inhalation solution in asthma have not been established.
2 DOSAGE AND ADMINISTRATION
The recommended dose of arformoterol tartrate inhalation solution is one 15 mcg unit-dose vial administered twice daily (morning and evening) by nebulization. A total daily dose of greater than 30 mcg (15 mcg twice daily) is not recommended.
Arformoterol tartrate inhalation solution should be administered by the orally inhaled route via a standard jet nebulizer connected to an air compressor (see the accompanying Patient Information). Arformoterol tartrate inhalation solution should not be swallowed. Arformoterol tartrate inhalation solution should be stored refrigerated in foil pouches. After opening the pouch, unused unit-dose vials should be returned to, and stored in, the pouch. An opened unit-dose vial should be used right away.
If the recommended maintenance treatment regimen fails to provide the usual response, medical advice should be sought immediately, as this is often a sign of destabilization of COPD. Under these circumstances, the therapeutic regimen should be reevaluated and additional therapeutic options should be considered.
No dose adjustment is required for patients with renal or hepatic impairment. However, since the clearance of arformoterol tartrate inhalation solution is prolonged in patients with hepatic impairment, they should be monitored closely.
The drug compatibility (physical and chemical), efficacy, and safety of arformoterol tartrate inhalation solution when mixed with other drugs in a nebulizer have not been established.
The safety and efficacy of arformoterol tartrate inhalation solution have been established in clinical trials when administered using the PARI LC® Plus nebulizer (with a face mask or mouthpiece) and the PARI DURA NEB™ 3,000 compressor. The safety and efficacy of arformoterol tartrate inhalation solution delivered from non-compressor based nebulizer systems have not been established.
3 DOSAGE FORMS AND STRENGTHS
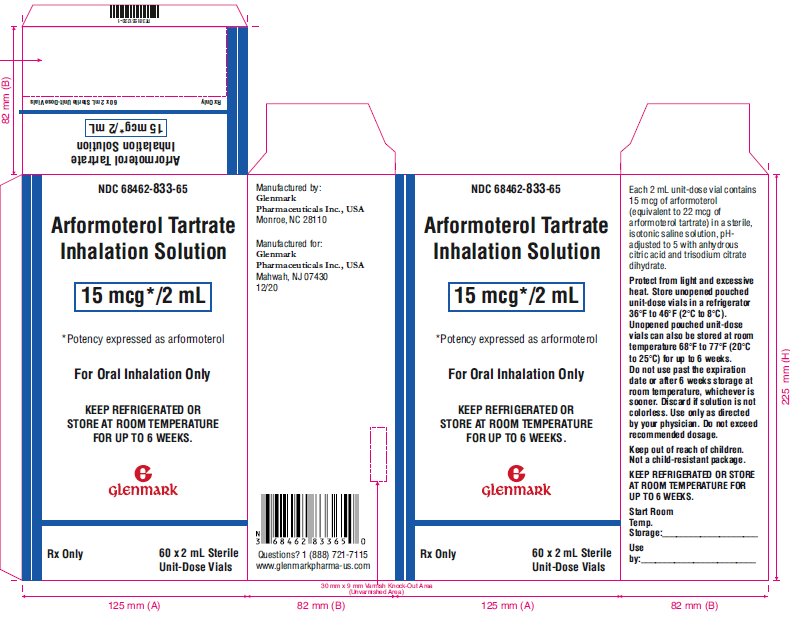
Arformoterol tartrate inhalation solution is supplied as a sterile solution for nebulization in low-density polyethylene unit-dose vials. Each 2 mL vial contains 15 mcg of arformoterol equivalent to 22 mcg of arformoterol tartrate.
4 CONTRAINDICATIONS
Arformoterol tartrate inhalation solution is contraindicated in patients with a history of hypersensitivity to arformoterol, racemic formoterol or to any other components of this product.
Use of a LABA, including arformoterol tartrate inhalation solution, without an inhaled cortisteroid is contraindicated in patients with asthma [see Warnings and Precautions (5)]. Arformoterol inhalation solution is not indicated for the treatment of asthma.
5 WARNINGS AND PRECAUTIONS
5.1 Serious Asthma-Related Events - Hospitalizations, Intubations, Deaths
- •
- The safety and efficacy of arformoterol tartrate inhalation solution in patients with asthma have not been established. Arformoterol tartrate inhalation solution is not indicated for the treatment of asthma [see Contraindications (4)].
- •
- Use of long-acting beta2-adrenergic agonists (LABA) as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, and death) compared with ICS alone.
- •
- A 28-week, placebo-controlled US study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol vs. 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of the LABA, including arformoterol tartrate inhalation solution.
- •
- No study adequate to determine whether the rate of asthma-related death is increased in patients treated with arformoterol tartrate inhalation solution has been conducted. Clinical studies with racemic formoterol suggested a higher incidence of serious asthma exacerbations in patients who received racemic formoterol than in those who received placebo. The sizes of these studies were not adequate to precisely quantify the differences in serious asthma exacerbation rates between treatment groups.
- •
- Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
5.2 Deterioration of Disease and Acute Episodes
Arformoterol tartrate inhalation solution should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. The use of arformoterol tartrate inhalation solution in this setting is inappropriate.
Arformoterol tartrate inhalation solution is not indicated for the treatment of acute episodes of bronchospasm, i.e., as rescue therapy and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta2-agonist.
When beginning arformoterol tartrate inhalation solution, patients who have been taking inhaled short-acting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing arformoterol tartrate inhalation solution, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient how it should be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated. COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If arformoterol tartrate inhalation solution no longer controls the symptoms of bronchoconstriction, or the patient’s inhaled, short-acting beta2-agonist becomes less effective or the patient needs more inhalation of short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, a reevaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dosage of arformoterol tartrate inhalation solution beyond the recommended 15 mcg twice daily dose is not appropriate in this situation.
5.3 Excessive Use of Arformoterol Tartrate Inhalation Solution and Use with Other Long-Acting Beta2-Agonists
Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. As with other inhaled beta2-adrenergic drugs, arformoterol tartrate inhalation solution should not be used more often, at higher doses than recommended, or in conjunction with other medications containing long-acting beta2-agonists.
5.4 Paradoxical Bronchospasm
As with other inhaled beta2-agonists, arformoterol tartrate inhalation solution can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs, arformoterol tartrate inhalation solution should be discontinued immediately and alternative therapy instituted.
5.5 Cardiovascular Effects
Arformoterol tartrate inhalation solution, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic and/or diastolic blood pressure, and/or symptoms. If such effects occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce ECG changes, such as flattening of the T-wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Arformoterol tartrate inhalation solution, as with other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
5.6 Coexisting Conditions
Arformoterol tartrate inhalation solution, like other sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension; in patients with convulsive disorders or thyrotoxicosis, and in patients who are unusually responsive to sympathomimetic amines. In two pooled, 12-week, placebo-controlled trials investigating arformoterol tartrate inhalation solution doses of 15 mcg BID, 25 mcg BID, and 50 mcg QD, changes in mean predose and 2-hour post dose systolic and/or diastolic blood pressure were seen as a general fall of 2 to 4 mm/Hg; for pulse rate the mean of maximal increases were 8.8 to 12 beats/min. Over the course of a one-year study measuring serial electrocardiograms while receiving a dose of 50 mcg daily of arformoterol tartrate inhalation solution resulted in an approximately 3 ms increase in QTC-Fcompared to the active comparator, salmeterol. Doses of the related beta2-agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
5.7 Hypokalemia and Hyperglycemia
Beta-agonist medications may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects [see Clinical Pharmacology (12.2)]. The decrease in serum potassium is usually transient, not requiring supplementation. Beta-agonist medications may produce transient hyperglycemia in some patients.
Clinically significant and dose-related changes in serum potassium and blood glucose were infrequent during clinical trials with long-term administration of arformoterol tartrate inhalation solution at the recommended dose.
6 ADVERSE REACTIONS
Long-acting beta2-adrenergic agonists, such as arformoterol tartrate, as monotherapy (without inhaled corticosteroids) for asthma increase the risk of asthma-related events. Arformoterol tartrate inhalation solution is not indicated for the treatment of asthma [see Warnings and Precautions (5.1)].
6.1 Beta2-Agonist Adverse Reaction Profile
Adverse reactions to arformoterol tartrate inhalation solution are expected to be similar in nature to other beta2-adrenergic receptor agonists including: angina, hypertension or hypotension, tachycardia, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, muscle cramps, nausea, dizziness, fatigue, malaise, hypokalemia, hyperglycemia, metabolic acidosis and insomnia.
6.2 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Adults with COPD in Short-Term Trials (12 weeks)
The safety data described below for adults ≥35 years of age are based on 2 clinical trials of 12 weeks. In the 2 trials of 12 weeks duration, 1456 patients (860 males and 596 females, ages 34 to 89 years old) with COPD were treated with arformoterol tartrate inhalation solution 15 mcg twice daily, 25 mcg twice daily, 50 mcg once daily, salmeterol 42 mcg twice daily, or placebo. The racial/ethnic distribution in these two trials included 1383 Caucasians, 49 Blacks, 10 Asians, and 10 Hispanics, and 4 patients classified as other.
Among the 1,456 COPD patients in two 12-week, placebo-controlled trials, 288 were treated with arformoterol tartrate inhalation solution 15 mcg twice daily and 293 were treated with placebo. Doses of 25 mcg twice daily and 50 mcg once daily were also evaluated.
Table 1 shows adverse reaction rates among patients from these two trials where the frequency was greater than or equal to 2% in the arformoterol tartrate inhalation solution 15 mcg twice daily group and where the rate in the arformoterol tartrate inhalation solution 15 mcg twice daily group exceeded the rate in the placebo group. The total number and percent of patients who reported adverse events were 202 (70%) in the 15 mcg twice daily and 219 (75%) in the placebo groups. Ten adverse events demonstrated a dose relationship: asthenia, fever, bronchitis, COPD, headache, vomiting, hyperkalemia, leukocytosis, nervousness, and tremor.
- Table 1:N um be r of P at ie nt s Ex per ie nc ing A dver se Eve nt s fr om Tw o 12- Wee k, D ouble- Blind , P lace bo-C ontr olle d Cl inic al Tr ials
|
|
|
||
|
|
|
|
|
|
|
293 |
|
|
|
|
|
|
|
|
|
|
|
(6) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- * Reported terms coded to “Lung Disorder” were predominantly pulmonary or chest congestion.
Adverse events occurring in patients treated with arformoterol tartrate inhalation solution 15 mcg twice daily with a frequency of < 2%, but greater than placebo, were as follows:
Body as a Whole: abscess, allergic reaction, digitalis intoxication, fever, hernia, injection site pain, neck rigidity, neoplasm, pelvic pain, retroperitoneal hemorrhage
Cardiovascular: arteriosclerosis, atrial flutter, AV block, congestive heart failure, heart block, myocardial infarct, QT interval prolonged, supraventricular tachycardia, inverted T-wave
Digestive: constipation, gastritis, melena, oral moniliasis, periodontal abscess, rectal hemorrhage
Metabolic and Nutritional Disorders: dehydration, edema, glucose tolerance decreased, gout, hyperglycemia, hyperlipemia, hypoglycemia, hypokalemia
Musculoskeletal: arthralgia, arthritis, bone disorder, rheumatoid arthritis, tendinous contracture
Nervous: agitation, cerebral infarct, circumoral paresthesia, hypokinesia, paralysis, somnolence, tremor
Respiratory: carcinoma of the lung, respiratory disorder, voice alteration
Skin and Appendages: dry skin, herpes simplex, herpes zoster, skin discoloration, skin hypertrophy
Special Senses: abnormal vision, glaucoma
Urogenital: breast neoplasm, calcium crystalluria, cystitis, glycosuria, hematuria, kidney calculus, nocturia, PSA increase, pyuria, urinary tract disorder, urine abnormality.
In these trials, the overall frequency of all cardiovascular adverse events was 6.9% in arformoterol tartrate inhalation solution 15 mcg twice daily and 13.3% in the placebo group. There were no frequently occurring specific cardiovascular adverse events for arformoterol tartrate inhalation solution (frequency ≥1% and greater than placebo). The rate of COPD exacerbations was also comparable between the arformoterol tartrate inhalation solution 15 mcg twice daily and placebo groups, 12.2% and 15.1%, respectively.
Adults with COPD in Long-Term (52-week) Safety Trial
Arformoterol tartrate inhalation solution was evaluated in one 52 week double-blind, randomized, placebo-controlled, safety trial conducted in patients with moderate to severe COPD. The primary endpoint was time to either respiratory death or first COPD exacerbation-related hospitalization, whichever occurred first. The event had to be a death or hospitalization for which the patient’s respiratory status was predominant and/or inciting contributor, as determined by the clinical investigator. The objective of the trial was to demonstrate that the risk of respiratory death or COPD exacerbation-related hospitalization for patients treated with arformoterol tartrate inhalation solution was not greater than 40% more than the risk for patient treated with placebo. A total of 841 patients (479 males and 361 females, ages 41 to 94 years old) with COPD were randomized: 420 to arformoterol tartrate inhalation solution 15 mcg twice daily and 421 to placebo. Of the randomized patients, 255 (61%) in the arformoterol tartrate inhalation solution group and 211 (50%) in the placebo group, completed one year of treatment. The trial objective was met demonstrating that COPD patients treated with arformoterol tartrate inhalation solution are not at an increased risk of respiratory death or COPD exacerbation-related hospitalizations compared to placebo.
7 DRUG INTERACTIONS
7.1 Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of arformoterol may be potentiated [see Warnings and Precautions (5.3, 5.5, 5.6, 5.7)].
7.2 Xanthine Derivatives, Steroids or Diuretics
Concomitant treatment with methylxanthine (aminophylline, theophylline), steroids, or diuretics may potentiate any hypokalemic effect of adrenergic agonists including arformoterol tartrate inhalation solution [see Warnings and Precautions (5.7)].
The concurrent use of intravenously or orally administered methylxanthines (e.g., aminophylline, theophylline) by patients receiving arformoterol tartrate inhalation solution has not been completely evaluated. In two combined 12-week, placebo-controlled trials that included arformoterol tartrate inhalation solution doses of 15 mcg twice daily, 25 mcg twice daily, and 50 mcg once daily, 54 of 873 arformoterol tartrate inhalation solution-treated subjects received concomitant theophylline at study entry. In a 12-month controlled trial that included a 50 mcg once daily arformoterol tartrate inhalation solution dose, 30 of the 528 arformoterol tartrate inhalation solution-treated subjects received concomitant theophylline at study entry. In these trials, heart rate and systolic blood pressure were approximately 2 to 3 bpm and 6 to 8 mm Hg higher, respectively, in subjects on concomitant theophylline compared with the overall population.
7.3 Non-potassium Sparing Diuretics
The ECG changes and/or hypokalemia that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, especially when the recommended dose of the beta-agonist is exceeded. Although the clinical significance of these effects is not known, caution is advised in the co‑ administration of beta-agonists, including arformoterol tartrate inhalation solution, with non-potassium sparing diuretics.
7.4 MAO Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
Arformoterol tartrate inhalation solution, as with other beta-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or drugs known to prolong the QTc interval because of the effect of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval have an increased risk of ventricular arrhythmias.
7.5 Beta-Blockers
Beta-adrenergic receptor antagonists (beta-blockers) and arformoterol tartrate inhalation solution may inhibit the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies in pregnant women. Arformoterol tartrate should only be used during pregnancy if the expected benefit to the patient outweighs the potential risk to the fetus. Women should be advised to contact their physician if they become pregnant while taking arformoterol tartrate. In animal reproduction studies with arformoterol administered by the oral route to rats and rabbits at exposures approximately 370 and 8,400 times the adult exposure at the maximum recommended human daily inhalation dose (MRHDID) of 15 mcg every 12 hours, respectively, there were findings of structural abnormalities, embryofetal and infant mortality, and alterations of growth. These adverse effects generally occurred at large multiples of the MRHDID when arformoterol was administered by the oral route to achieve high systemic exposures. No evidence of fetal harm was observed in rabbits at an exposure approximately 4,900 times the MRHDID.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Labor or Delivery:
The potential effect of arformoterol tartrate on labor and delivery is unknown. Because of the potential for beta-agonists interference with uterine contractility, use of arformoterol tartrate inhalation solution during labor should be restricted to whom the benefits clearly outweigh the risk.
Data
Animal Data
In an embryofetal development study in which pregnant rats received doses of 1,000, 5,000 or 10,000 mcg/kg/day from gestation days 6 to 17, arformoterol was shown to be teratogenic based upon findings of omphalocele (umbilical hernia), a malformation, in rat fetuses at exposures approximately 370 times adult exposure at the MRHDID (on an AUC basis with maternal oral doses of 1,000 mcg/kg/day and higher. Maternal toxicity was not observed in rats with exposures up to 2,400 times the MRHDID (on an AUC basis with maternal oral doses up to 10,000 mcg/kg/day). A no-observed-adverse-effect-level (NOAEL) for rat fetuses was not identified. In an embryofetal development study in which pregnant rabbits received doses of 20,000, 40,000 or 80,000 mcg/kg/day from gestation days 7 to 20, arformoterol was shown to be teratogenic based upon findings of malpositioned right kidney, a malformation, in rabbit fetuses at exposures approximately 8400 times and higher than the adult exposure at the MRHDID (on an AUC basis with maternal oral doses of 20,000 mcg/kg/day and higher). Malformations including brachydactyly, bulbous aorta, and liver cysts as well as decreased body weights were observed in rabbit fetuses at doses approximately 26,000 times and higher than the MRHDID in adults (on a mcg/m2 basis with maternal oral doses of 40,000 mcg/kg/day and higher). Malformations including adactyly, lobular dysgenesis of the lung, and interventricular septal defect as well as embryolethality were observed in rabbit fetuses at a dose approximately 52,000 times the MRHDID in adults (on a mcg/m2 basis with a maternal oral dose of 80,000 mcg/kg/day). Maternal toxicity was observed at doses approximately 26,000 times and higher than the MRHDID in adults (on a mcg/m2 basis with maternal oral doses of 40,000 mcg/kg/day and higher). There was no evidence of fetal harm in rabbits at exposures approximately 4,900 times and lower than the adult exposure at the MRHDID (on an AUC basis with maternal oral doses of 10,000 mcg/kg/day and lower).
In a pre- and post-natal development study, female rats received arformoterol at oral doses of 0, 1,000, 5,000, and 10,000 mcg/kg/day from gestation day 6 through lactation day 20. Lengths of gestation for female rats receiving doses 325 times and higher than the MRHDID (on a mcg/m2 basis with maternal oral doses of 1,000 mcg/kg/day and higher) were slightly prolonged, which was attributed to prolonged parturition or dystocia due to the pharmacological action of β-adrenergic agonists such as arformoterol to relax uterine musculature. One female that had received a dose 3,200 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 10,000 mcg/kg/day) was euthanized due to complications during parturition. Pup survival and body weights were decreased at doses 1,600 times and higher than the MRHDID (on a mcg/m2 basis with maternal oral doses of 5,000 mcg/kg/day and higher) at birth and during lactation. Umbilical hernia, a malformation, was observed for 1 pup at a dose 3,200 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 10,000 mcg/kg/day). Potential developmental delays of rat pups were observed at a dose 3,200 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 10,000 mcg/kg/day); however, no developmental delays were evident with doses 1,600 times the MRHDID (on a mcg/m2 basis with a maternal oral dose of 5,000 mcg/kg/day).
8.2 Lactation
Risk Summary
There are no data on the presence of arformoterol or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. However, arformoterol was excreted in the milk of lactating rats. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for arformoterol tartrate and any potential adverse effects on the breastfed infant from arformoterol tartrate or from the underlying maternal condition.
Data
Arformoterol and its metabolites were detected in the milk of lactating rats following oral administration of a 10,000 mcg/kg dose of radiolabeled arformoterol tartrate.
8.4 Pediatric Use
Arformoterol tartrate inhalation solution is approved for use in the long-term maintenance treatment of bronchoconstriction associated with chronic obstructive pulmonary disease, including chronic bronchitis and emphysema. This disease does not occur in children. The safety and efficacy of arformoterol tartrate inhalation solution in pediatric patients have not been established.
8.5 Geriatric Use
Of the 873 patients who received arformoterol tartrate inhalation solution in two placebo-controlled clinical studies in adults with COPD, 391 (45%) were 65 years of age or older while 96 (11%) were 75 years of age or older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Among subjects age 65 years and older, 129 (33%) received arformoterol tartrate inhalation solution at the recommended dose of 15 mcg twice daily, while the remainder received higher doses. ECG alerts for ventricular ectopy in patients 65 to ≤ 75 years of age were comparable among patients receiving 15 mcg twice daily, 25 mcg twice daily, and placebo (3.9%, 5.2%, and 7.1%, respectively). A higher frequency (12.4%) was observed when arformoterol tartrate inhalation solution was dosed at 50 mcg once daily. The clinical significance of this finding is not known. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Hepatic Impairment
Arformoterol tartrate inhalation solution should be used cautiously in patients with hepatic impairment due to increased systemic exposure in these patients [see Clinical Pharmacology (12.3)].
8.7 Renal Impairment
The systemic exposure to arformoterol was similar to renally impaired patients compared with demographically matched healthy control subjects [see Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
There were no reported cases of abuse or evidence of drug dependence with the use of arformoterol tartrate inhalation solution in the clinical trials.
10 OVERDOSAGE
The expected signs and symptoms associated with overdosage of arformoterol tartrate inhalation solution are those of excessive beta-adrenergic stimulation and/or occurrence or exaggeration of any of the signs and symptoms listed under ADVERSE REACTIONS. Signs and symptoms may include angina, hypertension or hypotension, tachycardia, with rates up to 200 beats/min, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, muscle cramps, nausea, dizziness, fatigue, malaise, hypokalemia, hyperglycemia, metabolic acidosis and insomnia. As with all inhaled sympathomimetic medications, cardiac arrest and even death may be associated with an overdose of arformoterol tartrate inhalation solution.
Treatment of overdosage consists of discontinuation of arformoterol tartrate inhalation solution together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. There is insufficient evidence to determine if dialysis is beneficial for overdosage of arformoterol tartrate inhalation solution. Cardiac monitoring is recommended in cases of overdosage.
11 DESCRIPTION
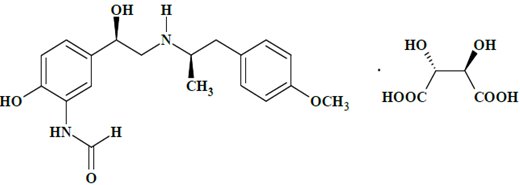
Arformoterol tartrate inhalation solution is a sterile, clear, colorless, aqueous solution of the tartrate salt of arformoterol, the (R,R)-enantiomer of formoterol. Arformoterol is a selective beta2-adrenergic bronchodilator. The chemical name for arformoterol tartrate is formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl] amino]ethyl]phenyl]-, (2R,3R)-2,3-dihydroxybutanedioate (1:1 salt), and its established structural formula is as follows:
The molecular weight of arformoterol tartrate is 494.49 g/mol, and its molecular formula is C19H24N2O4•C4H6O6 (1:1 salt). It is a white to off-white powder, freely soluble in dimethyl sulfoxide, sparingly soluble in water, slightly soluble in methanol, insoluble in acetonitrile.
Arformoterol tartrate is the United States Adopted Name (USAN) for (R,R)‑formoterol L-tartrate.
Arformoterol tartrate inhalation solution is supplied as 2 mL of arformoterol tartrate solution packaged in 2 mL unit-dose, low-density polyethylene (LDPE) unit-dose vials. Each unit-dose vial contains 15 mcg of arformoterol (equivalent to 22 mcg of arformoterol tartrate) in a sterile, isotonic saline solution, pH-adjusted to 5 with anhydrous citric acid and trisodium citrate dihydrate.
Arformoterol tartrate inhalation solution requires no dilution before administration by nebulization. Like all other nebulized treatments, the amount delivered to the lungs will depend upon patient factors, the nebulizer used, and compressor performance. Using the PARI LC® Plus nebulizer (with mouthpiece) connected to a PARI DURA NEB™ 3,000 compressor under in vitro conditions, the mean delivered dose from the mouthpiece (% nominal) was approximately 4.1 mcg (27.6%) at a mean flow rate of 3.3 L/min. The mean nebulization time was 6 minutes or less. Arformoterol tartrate inhalation solution should be administered from a standard jet nebulizer at adequate flow rates via face mask or mouthpiece.
Patients should be carefully instructed on the correct use of this drug product (please refer to the accompanying Patient Information).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Arformoterol, the (R,R)-enantiomer of formoterol, is a selective long-acting beta2‑adrenergic receptor agonist (beta2-agonist) that has two-fold greater potency than racemic formoterol (which contains both the (S,S) and (R,R)-enantiomers). The (S,S)-enantiomer is about 1,000-fold less potent as a beta2-agonist than the (R,R)-enantiomer. While it is recognized that beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors in the heart, data indicate that there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta2‑ adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including arformoterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3′,5′‑adenosine monophosphate (cyclic AMP). Increased intracellular cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
In vitro tests show that arformoterol is an inhibitor of the release of mast cell mediators, such as histamine and leukotrienes, from the human lung. Arformoterol also inhibits histamine-induced plasma albumin extravasation in anesthetized guinea pigs and inhibits allergen-induced eosinophil influx in dogs with airway hyper-responsiveness. The relevance of these in vitro and animal findings to humans is unknown.
12.2 Pharmacodynamics
Systemic Safety and Pharmacokinetic/Pharmacodynamic Relationships
The predominant adverse effects of inhaled beta2-agonists occur as a result of excessive activation of systemic beta-adrenergic receptors. The most common adverse effects may include skeletal muscle tremor and cramps, insomnia, tachycardia, decreases in plasma potassium, and increases in plasma glucose.
Effects on Serum Potassium and Serum Glucose Levels
Changes in serum potassium and serum glucose were evaluated in a dose-ranging study of twice daily (5 mcg, 15 mcg, or 25 mcg; 215 patients with COPD) and once daily (15 mcg, 25 mcg, or 50 mcg; 191 patients with COPD) arformoterol tartrate inhalation solution in COPD patients. At 2 and 6 hours post dose at week 0 (after the first dose), mean changes in serum potassium ranging from 0 to -0.3 mEq/L were observed in the arformoterol tartrate inhalation solution groups with similar changes observed after 2 weeks of treatment. Changes in mean serum glucose levels, ranging from a decrease of 1.2 mg/dL to an increase of 32.8 mg/dL were observed for arformoterol tartrate inhalation solution dose groups at both 2 and 6 hours post dose, both after the first dose and 14 days of daily treatment.
Electrophysiology
The effect of arformoterol tartrate inhalation solution on QT interval was evaluated in a dose-ranging study following multiple doses of arformoterol tartrate inhalation solution 5 mcg, 15 mcg, or 25 mcg twice daily or 15 mcg, 25 mcg, or 50 mcg once daily for 2 weeks in patients with COPD. ECG assessments were performed at baseline, time of peak plasma concentration and throughout the dosing interval. Different methods of correcting for heart rate were employed, including a subject-specific method and the Fridericia method.
Relative to placebo, the mean change in subject-specific QTc averaged over the dosing interval ranged from -1.8 to 2.7 msec, indicating little effect of arformoterol tartrate inhalation solution on cardiac repolarization after 2 weeks of treatment. The maximum mean change in subject-specific QTc for the arformoterol tartrate inhalation solution 15 mcg twice daily dose was 17.3 msec, compared with 15.4 msec in the placebo group. No apparent correlation of QTc with arformoterol plasma concentration was observed.
Electrocardiographic Monitoring in Patients with COPD
The effect of different doses of arformoterol tartrate inhalation solution on cardiac rhythm was assessed using 24-hour Holter monitoring in two 12-week, double-blind, placebo-controlled studies of 1,456 patients with COPD (873 received arformoterol tartrate inhalation solution at 15 or 25 mcg twice daily or 50 mcg once daily doses; 293 received placebo; 290 received salmeterol). The 24-hour Holter monitoring occurred once at baseline, and up to 3 times during the 12-week treatment period. The rates of new-onset cardiac arrhythmias not present at baseline over the double-blind 12-week treatment period were similar (approximately 33 to 34%) for patients who received arformoterol tartrate inhalation solution 15 mcg twice daily to those who received placebo. There was a dose-related increase in new, treatment-emergent arrhythmias seen in patients who received arformoterol tartrate inhalation solution 25 mcg twice daily and 50 mcg once daily, 37.6% and 40.1%, respectively. The frequencies of new treatment-emergent events of non-sustained (3 to 10 beat run) and sustained (>10 beat run) ventricular tachycardia were 7.4% and 1.1% in arformoterol tartrate nhalation solution 15 mcg twice daily and 6.9% and 1% in placebo. In patients who received arformoterol tartrate inhalation Solution 25 mcg twice daily and 50 mcg once daily, the frequencies of non-sustained (6.2% and 8.2%, respectively) and sustained ventricular tachycardia (1% and 1%, respectively) were similar. Five cases of ventricular tachycardia were reported as adverse events (1 in arformoterol tartrate inhalation solution 15 mcg twice daily and 4 in placebo), with two of these events leading to discontinuation of treatment (2 in placebo).
There were no baseline occurrences of atrial fibrillation/flutter observed on 24-hour Holter monitoring in patients treated with arformoterol tartrate inhalation solution 15 mcg twice daily or placebo. New, treatment-emergent atrial fibrillation/flutter occurred in 0.4% of patients who received arformoterol tartrate inhalation solution 15 mcg twice daily and 0.3% of patients who received placebo. There was a dose-related increase in the frequency of atrial fibrillation/flutter reported in the arformoterol tartrate inhalation solution 25 mcg twice daily and 50 mcg once daily dose groups of 0.7% and 1.4%, respectively. Two cases of atrial fibrillation/flutter were reported as adverse events (1 in arformoterol tartrate inhalation solution 15 mcg twice daily and 1 in placebo).
Dose-related increases in mean maximum change in heart rate in the 12 hours after dosing were also observed following 12 weeks of dosing with arformoterol tartrate inhalation solution 15 mcg twice daily (8.8 bpm), 25 mcg twice daily (9.9 bpm) and 50 mcg once daily (12 bpm) versus placebo (8.5 bpm).
Tachyphylaxis/Tolerance
Tolerance to the effects of inhaled beta-agonists can occur with regularly-scheduled, chronic use.
In two placebo-controlled clinical trials in patients with COPD involving approximately 725 patients in each, the overall efficacy of arformoterol tartrate inhalation solution was maintained throughout the 12-week trial duration. However, tolerance to the bronchodilator effect of arformoterol tartrate inhalation solution was observed after 6 weeks of dosing, as measured by a decrease in trough FEV1. FEV1 improvement at the end of the 12‑ hour dosing interval decreased by approximately one-third (22.1% mean improvement after the first dose compared to 14.6% at week 12). Tolerance to the trough FEV1 bronchodilator effect of arformoterol tartrate inhalation solution was not accompanied by other clinical manifestations of tolerance in these trials.
12.3 Pharmacokinetics
The pharmacokinetics (PK) of arformoterol have been investigated in healthy subjects, elderly subjects, renally and hepatically impaired subjects, and COPD patients following the nebulization of the recommended therapeutic dose and doses up to 96 mcg.
Absorption
In COPD patients administered 15 mcg arformoterol every 12 hours for 14 days, the mean steady-state peak (R,R)-formoterol plasma concentration (Cmax) and systemic exposure (AUC0 to 12h) were 4.3 pg/mL and 34.5 pg•hr/mL, respectively. The median steady-state peak (R,R)-formoterol plasma concentration time (tmax) was observed approximately one-half hour after drug administration.
Systemic exposure to (R,R)-formoterol increased linearly with dose in COPD patients following arformoterol doses of 5 mcg, 15 mcg, or 25 mcg twice daily for 2 weeks or 15 mcg, 25 mcg, or 50 mcg once daily for 2 weeks.
In a crossover study in patients with COPD, when arformoterol 15 mcg inhalation solution and 12 and 24 mcg formoterol fumarate inhalation powder (Foradil® Aerolizer®) was administered twice daily for 2 weeks, the accumulation index was approximately 2.5 based on the plasma (R,R)-formoterol concentrations in all three treatments. At steady- state, geometric means of systemic exposure (AUC0 to 12h) to (R,R)-formoterol following 15 mcg of arformoterol inhalation solution and 12 mcg of formoterol fumarate inhalation powder were 39.33 pg•hr/mL and 33.93 pg•hr/mL, respectively (ratio 1.16; 90% CI 1.00, 1.35), while the geometric means of the Cmax were 4.30 pg/mL and 4.75 pg/mL, respectively (ratio 0.91; 90% CI 0.76, 1.09).
In a study in patients with asthma, treatment with arformoterol 50 mcg with pre- and post-treatment with activated charcoal resulted in a geometric mean decrease in (R,R)-formoterol AUC0 to 6h by 27% and Cmax by 23% as compared to treatment with arformoterol 50 mcg alone. This suggests that a substantial portion of systemic drug exposure is due to pulmonary absorption.
Distribution
The binding of arformoterol to human plasma proteins in vitro was 52 to 65% at concentrations of 0.25, 0.5 and 1 ng/mL of radiolabeled arformoterol. The concentrations of arformoterol used to assess the plasma protein binding were higher than those achieved in plasma following inhalation of multiple doses of 50 mcg arformoterol.
Metabolism
In vitro profiling studies in hepatocytes and liver microsomes have shown that arformoterol is primarily metabolized by direct conjugation (glucuronidation) and secondarily by O-demethylation. At least five human uridine diphosphoglucuronosyltransferase (UGT) isozymes catalyze arformoterol glucuronidation in vitro. Two cytochrome P450 isozymes (CYP2D6 and secondarily CYP2C19) catalyze the O-demethylation of arformoterol.
Arformoterol was almost entirely metabolized following oral administration of 35 mcg of radiolabeled arformoterol in eight healthy subjects. Direct conjugation of arformoterol with glucuronic acid was the major metabolic pathway. Most of the drug-related material in plasma and urine was in the form of glucuronide or sulfate conjugates of arformoterol. O-Desmethylation and conjugates of the O-desmethyl metabolite were relatively minor metabolites accounting for less than 17% of the dose recovered in urine and feces.
Elimination
After administration of a single oral dose of radiolabeled arformoterol to eight healthy male subjects, 63% of the total radioactive dose was recovered in urine and 11% in feces within 48 hours. A total of 89% of the total radioactive dose was recovered within 14 days, with 67% in urine and 22% in feces. Approximately 1% of the dose was recovered as unchanged arformoterol in urine over 14 days. Renal clearance was 8.9 L/hr for unchanged arformoterol in these subjects.
In COPD patients given 15 mcg inhaled arformoterol twice a day for 14 days, the mean terminal half-life of arformoterol was 26 hours.
Special Populations:
Gender
A population PK analysis indicated that there was no effect of gender upon the pharmacokinetics of arformoterol.
Race
The influence of race on arformoterol pharmacokinetics was assessed using a population PK analysis and data from healthy subjects. There was no clinically significant impact of race upon the pharmacokinetic profile of arformoterol.
Geriatric
The pharmacokinetic profile of arformoterol in 24 elderly subjects (aged 65 years or older) was compared to a younger cohort of 24 subjects (18 to 45 years) that were matched for body weight and gender. No significant differences in systemic exposure (AUC and Cmax) were observed when the two groups were compared.
Pediatric
The pharmacokinetics of arformoterol have not been studied in pediatric subjects.
Hepatic Impairment
The pharmacokinetic profile of arformoterol was assessed in 24 subjects with mild, moderate, and severe hepatic impairment. The systemic exposure (Cmax and AUC) to arformoterol increased 1.3 to 2.4-fold in subjects with hepatic impairment compared to 16 demographically matched healthy control subjects. No clear relationship between drug exposure and the severity of hepatic impairment was observed. Arformoterol tartrate inhalation solution should be used cautiously in patients with hepatic impairment.
Renal Impairment
The impact of renal disease upon the pharmacokinetics of arformoterol was studied in 24 subjects with mild, moderate, or severe renal impairment. Systemic exposure (AUC and Cmax) to arformoterol was similar in renally impaired patients compared with demographically matched healthy control subjects.
Drug-Drug Interaction
When paroxetine, a potent inhibitor of CYP2D6, was co-administered with arformoterol tartrate inhalation solution at steady-state, exposure to either drug was not altered. Dosage adjustments of arformoterol tartrate inhalation solution are not necessary when the drug is given concomitantly with potent CYP2D6 inhibitors.
Arformoterol did not inhibit CYP1A2, CYP2A6, CYP2C9/10, CYP2C19, CYP2D6, CYP2E1, CYP3A4/5, or CYP4A9/11 enzymes at >1,000-fold higher concentrations than the expected peak plasma concentrations following a therapeutic dose.
12.5 Pharmacogenomics
Arformoterol is eliminated through the action of multiple drug metabolizing enzymes.
Direct glucuronidation of arformoterol is mediated by several UGT enzymes and is the primary elimination route. O-Desmethylation is a secondary route catalyzed by the CYP enzymes CYP2D6 and CYP2C19. In otherwise healthy subjects with reduced CYP2D6 and/or UGT1A1 enzyme activity, there was no impact on systemic exposure to arformoterol compared to subjects with normal CYP2D6 and/or UGT1A1 enzyme activities.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies were conducted in mice using oral administration and rats using inhalation administration to evaluate the carcinogenic potential of arformoterol.
In a 24-month carcinogenicity study in CD-1 mice, arformoterol caused a dose-related increase in the incidence of uterine and cervical endometrial stromal polyps and stromal cell sarcoma in female mice at oral doses of 1,000 mcg/kg and above (AUC exposure approximately 70 times adult exposure at the MRHDID).
In a 24-month carcinogenicity study in Sprague-Dawley rats, arformoterol caused a statistically significant increase in the incidence of thyroid gland c-cell adenoma and carcinoma in female rats at an inhalation dose of 200 mcg/kg (AUC exposure approximately 130 times adult exposure at the MRHDID). There were no tumor findings with an inhalation dose of 40 mcg/kg (AUC exposure approximately 55 times adult exposure at the MRHDID).
Arformoterol was not mutagenic or clastogenic in the following tests: mutagenicity tests in bacteria, chromosome aberration analyses in mammalian cells, and micronucleus test in mice.
Arformoterol had no effects on fertility and reproductive performance in rats at oral doses up to 10,000 mcg/kg (approximately 3,200 times the MRHDID in adults on a mcg/m2 basis).
13.2 Animal Toxicology and/or Pharmacology
Animal Pharmacology
In animal studies investigating its cardiovascular effects, arformoterol induced dose- dependent increases in heart rate and decreases in blood pressure consistent with its pharmacology as a beta-adrenergic agonist. In dogs, at systemic exposures higher than anticipated clinically, arformoterol also induced exaggerated pharmacologic effects of a beta‑ adrenergic agonist on cardiac function as measured by electrocardiogram (sinus tachycardia, atrial premature beats, ventricular escape beats, PVCs).
Studies in laboratory animals (minipigs, rodents, and dogs) have demonstrated the occurrence of arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines are administered concurrently. The clinical significance of these findings is unknown.
unknown.
14 CLINICAL STUDIES
14.1 Adult COPD Trials
Arformoterol tartrate inhalation solution was studied in two identical, 12-week, double-blind, placebo- and active-controlled, randomized, multi-center, parallel group trials conducted in the United States (Clinical Trial A and Clinical Trial B). A total of 1,456 adult patients (age range: 34 to 89 years; mean age: 63 years; gender: 860 males and 596 females) with COPD who had a mean FEV1 of 1.3 L (42% of predicted) were enrolled in the two clinical trials. The racial/ethnic distribution in these two trials included 1383 Caucasians, 49 Blacks, 10 Asians, and 10 Hispanics, and 4 patients classified as Other. The diagnosis of COPD was based on a prior clinical diagnosis of COPD, a smoking history (greater than 15 pack-years), age (at least 35 years), spirometry results (baseline FEV1 ≤ 65% of predicted value and > 0.70 L, and a FEV1/forced vital capacity (FVC) ratio ≤ 70%). About 80% of patients in these studies had bronchodilator reversibility, defined as a 10% or greater increase in FEV1 after inhalation of 2 actuations (180 mcg racemic albuterol from a metered dose inhaler). Both trials compared arformoterol tartrate inhalation solution 15 mcg twice daily (288 patients), 25 mcg twice daily (292 patients), 50 mcg once daily (293 patients) with placebo (293 subjects). Both trials included salmeterol inhalation aerosol, 42 mcg twice daily as an active comparator (290 patients).
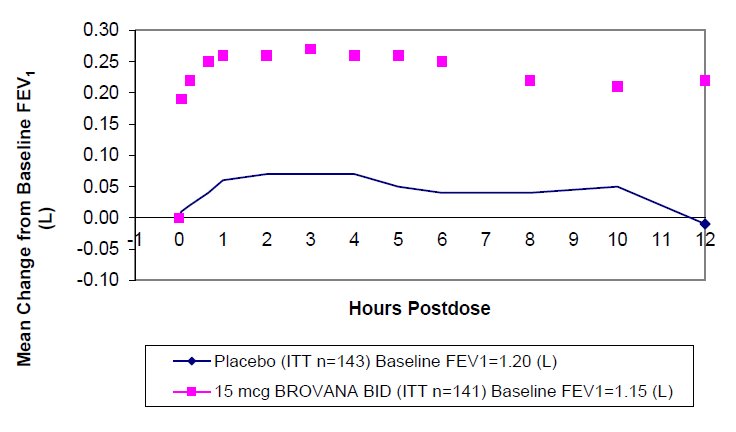
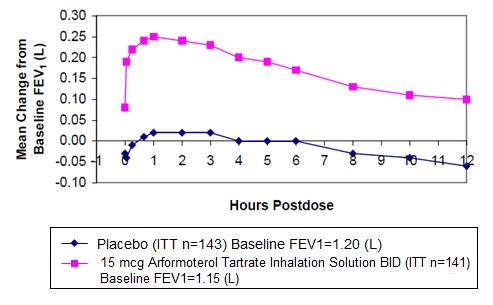
In both 12-week trials, arformoterol tartrate inhalation solution 15 mcg twice daily resulted in a statistically significant change of approximately 11% in mean FEV1 (as measured by percent change from study baseline FEV1 at the end of the dosing interval over the 12 weeks of treatment, the primary efficacy endpoint) compared to placebo. Compared to arformoterol tartrate inhalation solution 15 mcg twice daily, arformoterol tartrate inhalation solution 25 mcg twice daily and 50 mcg once daily did not provide sufficient additional benefit on a variety of endpoints, including FEV1, to support the use of higher doses. Plots of the mean change in FEV1 values obtained over the 12 hours after dosing for the arformoterol tartrate inhalation solution 15 mcg twice daily dose group and for the placebo group are provided in Figures 1 and 2 for Clinical Trial A, below. The plots include mean FEV1 change observed after the first dose and after 12 weeks of treatment. The results from Clinical Trial B were similar.
- Figure 1Me an C hange in FEV1 Ove r Tim e f or C linic al Tr ial A at Wee k 0 (D ay 1)
- Figure 2Me an C hange in FEV1 Ove r Tim e f or C linic al Tr ial A at Wee k 12
Arformoterol tartrate inhalation solution 15 mcg twice daily significantly improved bronchodilation compared to placebo over the 12 hours after dosing (FEV1 AUC0 to 12h). This improvement was maintained over the 12-week study period.
Following the first dose of arformoterol tartrate inhalation solution 15 mcg, the median time to onset of bronchodilation, defined by an FEV1 increase of 15%, occurred at 6.7 min. When defined as an increase in FEV1 of 12% and 200 mL, the time to onset of bronchodilation was 20 min after dosing. Peak bronchodilator effect was generally seen within 1 to 3 hours of dosing.
In both clinical trials, compared to placebo, patients treated with arformoterol tartrate inhalation solution demonstrated improvements in peak expiratory flow rates, supplemental ipratropium and rescue albuterol use.
16 HOW SUPPLIED/STORAGE AND HANDLING
Arformoterol tartrate inhalation solution is supplied in a single strength (15 mcg of arformoterol, equivalent to 22 mcg of arformoterol tartrate) as 2 mL of a sterile solution in low-density polyethylene (LDPE) unit-dose vials overwrapped in foil. Arformoterol tartrate inhalation solution is available in a shelf-carton containing 30 or 60 unit-dose vials.
NDC 68462-833-30: carton of 30 individually pouched unit-dose vials
NDC 68462-833-35: carton of 30 unit-dose vials (6×5 unit-dose vial pouches)
NDC 68462-833-60: carton of 60 unit-dose vials (15×4 unit-dose vial pouches)
NDC 68462-833-65: carton of 60 unit-dose vials (12×5 unit-dose vial pouches)
Storage and Handling
Store arformoterol tartrate inhalation solution in the protective foil pouch under refrigeration at 36°F to 46°F (2°C to 8°C). Protect from light and excessive heat. After opening the pouch, unused unit-dose vials should be returned to, and stored in, the pouch. An opened unit-dose vial should be used right away. Discard any unit-dose vial if the solution is not colorless. Unopened foil pouches of arformoterol tartrate inhalation solution can also be stored at room temperature 68°F to 77°F (20°C to 25°C) [see USP Controlled Room Temperature] for up to 6 weeks. If stored at room temperature, discard if not used after 6 weeks or if past the expiration date, whichever is sooner.
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use) with each new prescription and refill.
The complete text of the Patient Information is reprinted at the end of this document. Patients should be given the following information:
Serious Asthma-Related Events, Acute Exacerbations or Deteriorations
Patients should be informed that long-acting beta2-adrenergic agonists, such as arformoterol tartrate inhalation solution, when used as monotherapy [without an inhaled corticosteroid], increase risk of serious asthma-related events, including asthma-related death. Arformoterol inhalation solution is not indicated for the treatment of asthma.
Arformoterol tartrate inhalation solution is not indicated to relieve acute respiratory symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled, short-acting beta2-agonist (the healthcare provider should prescribe the patient with such medication and instruct the patient in how it should be used). Patients should be instructed to seek medical attention if their symptoms worsen despite recommended doses of arformoterol tartrate inhalation solution, if arformoterol tartrate inhalation solution treatment becomes less effective, or if they need more inhalations of a short-acting beta2‑ agonist than usual.
Appropriate Dosing
Patients should not stop using arformoterol tartrate inhalation solution unless told to do so by a healthcare provider because symptoms may get worse. Patients should not inhale more than one dose at any one time. The daily dosage of arformoterol tartrate inhalation solution should not exceed one unit-dose vial (15 mcg) by inhalation twice daily (30 mcg total daily dose). Excessive use of sympathomimetics may cause significant cardiovascular effects, and may be fatal.
Concomitant Therapy
Patients who have been taking inhaled, short-acting beta2-agonists (e.g., levalbuterol) on a regular basis should be instructed to discontinue the regular use of these products and use them only for the symptomatic relief of acute symptoms.
Arformoterol tartrate inhalation solution should not be used in conjunction with other inhaled medications containing long-acting beta2-agonists. Patients should be warned not to stop or change the dose of other concomitant COPD therapy without medical advice, even if symptoms improve after initiating treatment with arformoterol tartrate inhalation solution.
Common Adverse Reactions with Beta2-agonists
Patients should be informed that treatment with beta2-agonists may lead to adverse reactions that include palpitations, chest pain, rapid heart rate, increased or decreased blood pressure, headache, tremor, nervousness, dry mouth, muscle cramps, nausea, dizziness, fatigue, malaise, low blood potassium, high blood sugar, high blood acid, or trouble sleeping [see Adverse Reactions (Error! Hyperlink reference not valid.)].
Instructions for Administration
It is important that patients understand how to use arformoterol tartrate inhalation solution with a nebulizer appropriately and how it should be used in relation to other medications to treat COPD they are taking [see the accompanying Patient Information]. Patients should be instructed not to mix other medications with arformoterol tartrate inhalation solution and not to inject or swallow arformoterol tartrate inhalation solution. Patients should throw the plastic dispensing vials away immediately after use. Due to their small size, the vials pose a danger of choking to young children.
Women should be advised to contact their physician if they become pregnant or if they are nursing.
FDA-Approved Patient Information
See the accompanying Patient Information.
Manufactured by:
Glenmark Pharmaceuticals Inc., USA
Monroe, NC 28110
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
December 2020
PATIENT INFORMATION
Arformoterol Tartrate [AR-for-MOE-ter-ol TAR-trate]
Inhalation Solution
What is arformoterol tartrate inhalation solution?
- •
- Arformoterol tartrate inhalation solution is for long term use and should be taken 2 times each day (morning and evening), to help control the symptoms of chronic obstructive pulmonary disease (COPD) for better breathing.
- •
- COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- •
- Arformoterol tartrate inhalation solution is only for use with a nebulizer.
- •
- Long acting beta2 adrenergic agonist (LABA) medicines, such as arformoterol tartrate help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath.
- •
- Arformoterol tartrate inhalation solution is not used to treat sudden symptoms of COPD. Always have a short-acting beta2-agonist medicine (rescue inhaler) with you to treat sudden symptoms of COPD. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you.
- •
- Arformoterol tartrate inhalation solution is not for the treatment of asthma. It is not known if arformoterol tartrate is safe and effective in people with asthma.
- •
- Arformoterol tartrate inhalation solution should not be used in children. It is not known if arformoterol tartrate is safe and effective in children.
Do not use arformoterol tartrate inhalation solution if you:
- •
- are allergic to arformoterol, racemic formoterol, or any of the ingredients in arformoterol tartrate inhalation solution. Ask your healthcare provider if you are not sure. See the end of this leaflet for a complete list of ingredients in arformoterol tartrate inhalation solution.
- •
- have asthma.
Before you use arformoterol tartrate inhalation solution, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have heart problems
- •
- have high blood pressure
- •
- have seizures
- •
- have thyroid problems
- •
- have diabetes
- •
- have liver problems
- •
- are pregnant or plan to become pregnant. It is not known if arformoterol tartrate inhalation solution can harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed.It is not known if the arformoterol tartrate or any other ingredients in arformoterol tartrate inhalation solution passes into your milk and if it can harm your baby. You and your healthcare provider should decide if you will take arformoterol tartrate inhalation solution or breastfeed.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins and herbal supplements. Arformoterol tartrate inhalation solution and certain other medicines may interact with each other. This may cause serious side effects.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
How should I use arformoterol tartrate inhalation solution?
Read the step-by-step Instructions for Use for arformoterol tartrate inhalation solution at the end of this
Patient Information leaflet.
- •
- Use arformoterol tartrate inhalation solution exactly as prescribed. Do not use arformoterol tartrate inhalation solution more often than prescribed.
- •
- One unit-dose vial of arformoterol tartrate inhalation solution is 1 dose. The usual dose of arformoterol tartrate inhalation solution is 1 unit-dose vial, 2 times a day (morning and evening) breathed in through your nebulizer machine. The 2 doses should be taken about 12 hours apart. Do not use more than 2 unit-dose vials of arformoterol tartrate inhalation solution a day.
- •
- Do not swallow or inject arformoterol tartrate inhalation solution.
- •
- Arformoterol tartrate inhalation solution is for use with a standard jet nebulizer machine connected to an air compressor. Read the complete instructions for use at the end of this Patient Information leaflet before starting arformoterol tartrate inhalation solution.
- •
- Do not mix other medicines with arformoterol tartrate inhalation solution in your nebulizer machine.
- •
- While you are using arformoterol tartrate inhalation solution 2 times each day:
- •
- Do not use other medicines that contain a long-acting beta2-agonist (LABA) for any reason.
- •
- Do not use your short-acting beta2-agonist medicine on a regular basis (four times a day).
- •
- Arformoterol tartrate inhalation solution does not relieve sudden symptoms of COPD. Always have a rescue inhaler medicine with you to treat sudden symptoms. If you do not have a rescue inhaler medicine, call your healthcare provider to have one prescribed for you.
- •
- Do not stop using arformoterol tartrate inhalation solution or other medicines to control or treat your COPD unless told to do so by your healthcare provider because your symptoms might get worse. Your healthcare provider will change your medicines as needed.
Call your healthcare provider or get emergency medical care right away if your breathing problems worsen with arformoterol tartrate inhalation solution, you need to use your rescue medicine more often than usual, or your rescue inhaler medicine does not work as well for you at relieving symptoms.
What are the possible side effects of arformoterol tartrate inhalation solution?
Arformoterol tartrate inhalation solution can cause serious side effects, including:
- •
-
people with asthma who take long-acting beta2-adrenergic agonist (LABA) medicines, such as arformoterol (the medicine in arformoterol tartrate inhalation solution), without also using a medicine called an inhaled corticosteroid have an increased risk of serious problems from asthma, including being hospitalized, needing a tube placed in their airway to help them breath, or death.
- •
- Call your healthcare provider if breathing problems worsen over time while using arformoterol tartrate inhalation solution. You may need a different treatment.
- •
-
Get emergency medical care if:
- •
- your breathing problems worsen quickly.
- •
- you use a rescue inhaler medicine, but it does not relieve your breathing problems.
- •
- COPD symptoms that get worse over time. If your COPD symptoms worsen over time, do not increase your dose of arformoterol tartrate inhalation solution, instead call your healthcare provider.
- •
- using too much of a LABA medicine may cause:
|
|
- •
- sudden shortness of breath immediately after use of arformoterol tartrate inhalation solution. Sudden shortness of breath may be life threating. If you have sudden breathing problems immediately after inhaling your medicine, stop taking arformoterol tartrate inhalation solution and call your healthcare provider or go to the nearest hospital emergency room right away.
- •
- effects on the heart which may include:
|
|
- •
- serious allergic reactions including rash, hives, swelling of the face, mouth, and tongue, and breathing problems. Call your healthcare provider or get emergency medical care if you get any symptoms of a serious allergic reaction.
- •
- Changes in laboratory levels, including high levels of blood sugar (hyperglycemia) and low levels of potassium (hypokalemia).
Common side effects of arformoterol tartrate inhalation solution include:
|
|
|
|
Tell your healthcare provider if you get any side effect that bothers you or that does not go away.
These are not all the possible side effects of arformoterol tartrate inhalation solution. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store arformoterol tartrate inhalation solution?
- •
- Store arformoterol tartrate inhalation solution in a refrigerator between 36°F to 46°F (2°C to 8°C) in the protective foil pouch. Protect from light and excessive heat. Do not open a sealed pouch until you are ready to use a dose of arformoterol tartrate inhalation solution. After opening the pouch, unused unit-dose vials should be returned to, and stored in, the pouch. An opened unit-dose vial should be used right away.
- •
- Arformoterol tartrate inhalation solution may also be stored at room temperature between 68ºF to 77ºF (20ºC to 25ºC) for up to 6 weeks (42 days). If stored at room temperature, discard arformoterol tartrate inhalation solution if it is not used after 6 weeks or if past the expiration date, whichever is sooner. Space is provided on the packaging to record room temperature storage times.
- •
- Do not use arformoterol tartrate inhalation solution after the expiration date provided on the foil pouch and unit-dose vial.
- •
- Arformoterol tartrate inhalation solution should be colorless. Throw away (discard) arformoterol tartrate inhalation solution if it is not colorless.
- •
- Keep arformoterol tartrate inhalation solution and all medicines out of the reach of children.
General information about the safe and effective use of arformoterol tartrate inhalation solution.
Medicines are sometimes prescribed for purposes not mentioned in this Patient Information leaflet. Do not use arformoterol tartrate inhalation solution for a condition for which it was not prescribed. Do not give arformoterol tartrate inhalation solution to other people, even if they have the same symptoms that you may have. It may harm them.
You can ask your pharmacist or healthcare provider for information about arformoterol tartrate inhalation solution that was written for health professionals.
What are the ingredients in arformoterol tartrate inhalation solution?
Active ingredient: arformoterol tartrate
Inactive ingredients: anhydrous citric acid, sodium chloride, trisodium citrate dehydrate and water for injection.
Manufactured by:
Glenmark Pharmaceuticals Inc., USA
Monroe, NC 28110
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
December 2020
Instructions for Using Arformoterol Tartrate [AR-for-MOE-ter-ol TAR-trate] Inhalation Solution
Arformoterol tartrate inhalation solution is used only in a standard jet nebulizer machine connected to an air compressor. Make sure you know how to use your nebulizer machine before you use it to breathe in arformoterol tartrate inhalation solution or other medicines.
Do not mix arformoterol tartrate inhalation solution with other medicines in your nebulizer machine.
Arformoterol tartrate inhalation solution comes sealed in a foil pouch. Do not open a sealed pouch until you are ready to use a dose of arformoterol tartrate inhalation solution. After opening the pouch, unused unit-dose vials should be returned to, and stored in, the pouch. An opened unit-dose vial should be used right away.
- 1.
- Open the foil pouch by tearing on the rough edge along the seam of the pouch. Remove a unit-dose vial of arformoterol tartrate inhalation solution.
- 2.
- Carefully twist open the top of the unit-dose vial and use it right away (Figure 1).
Figure 1
- 1.
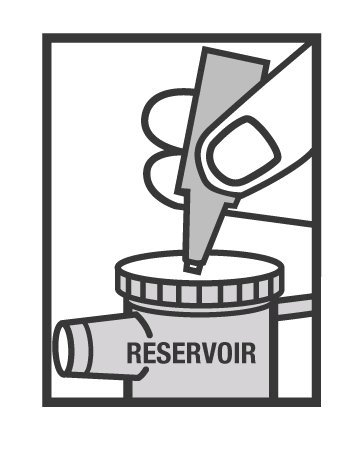
- Squeeze all of the medicine from the unit-dose vial into the nebulizer medicine cup (reservoir) (Figure 2).
Figure 2
- 1.
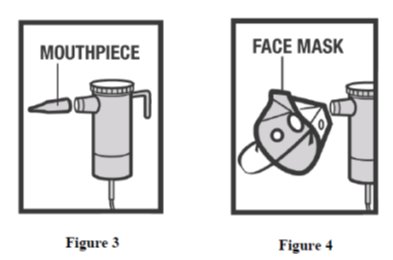
- Connect the nebulizer reservoir to the mouthpiece (Figure 3) or face mask (Figure 4).
- 1.
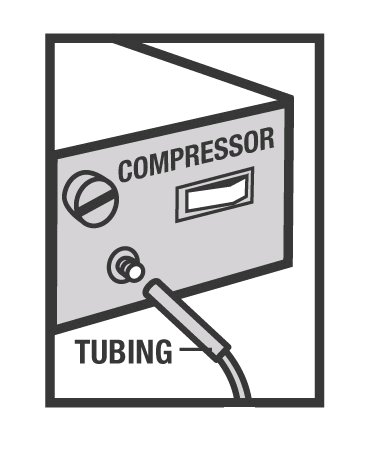
- Connect the nebulizer to the compressor (Figure 5).
Figure 5
- 1.
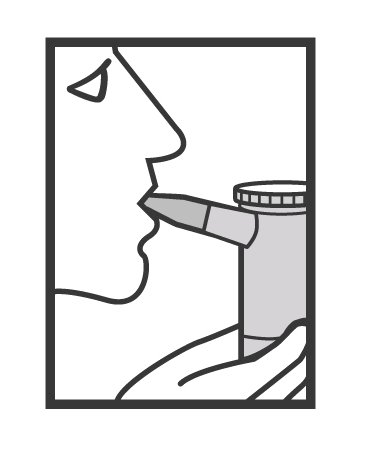
- Sit in a comfortable, upright position. Place the mouthpiece in your mouth (Figure 6) (or put on the face mask) and turn on the compressor.
Figure 6
- 1.
- Breathe as calmly, deeply, and evenly as possible until no more mist is formed in the nebulizer reservoir. It takes about 5 to 10 minutes for each treatment.
- 2.
- Clean the nebulizer (see manufacturer’s instructions).
Rx Only
This Instructions for Use has been approved by the Food and Drug Administration.
Manufactured by:
Glenmark Pharmaceuticals Inc., USA
Monroe, NC 28110
Manufactured for:
Glenmark Pharmaceuticals Inc., USA
Mahwah, NJ 07430
Questions? 1 (888) 721-7115
www.glenmarkpharma-us.com
December 2020
| ARFORMOTEROL TARTRATE
arformoterol tartrate solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - GLENMARK PHARMACEUTICALS INC., USA (130597813) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLENMARK PHARMACEUTICALS INC., USA | 079922908 | MANUFACTURE(68462-833) , ANALYSIS(68462-833) | |