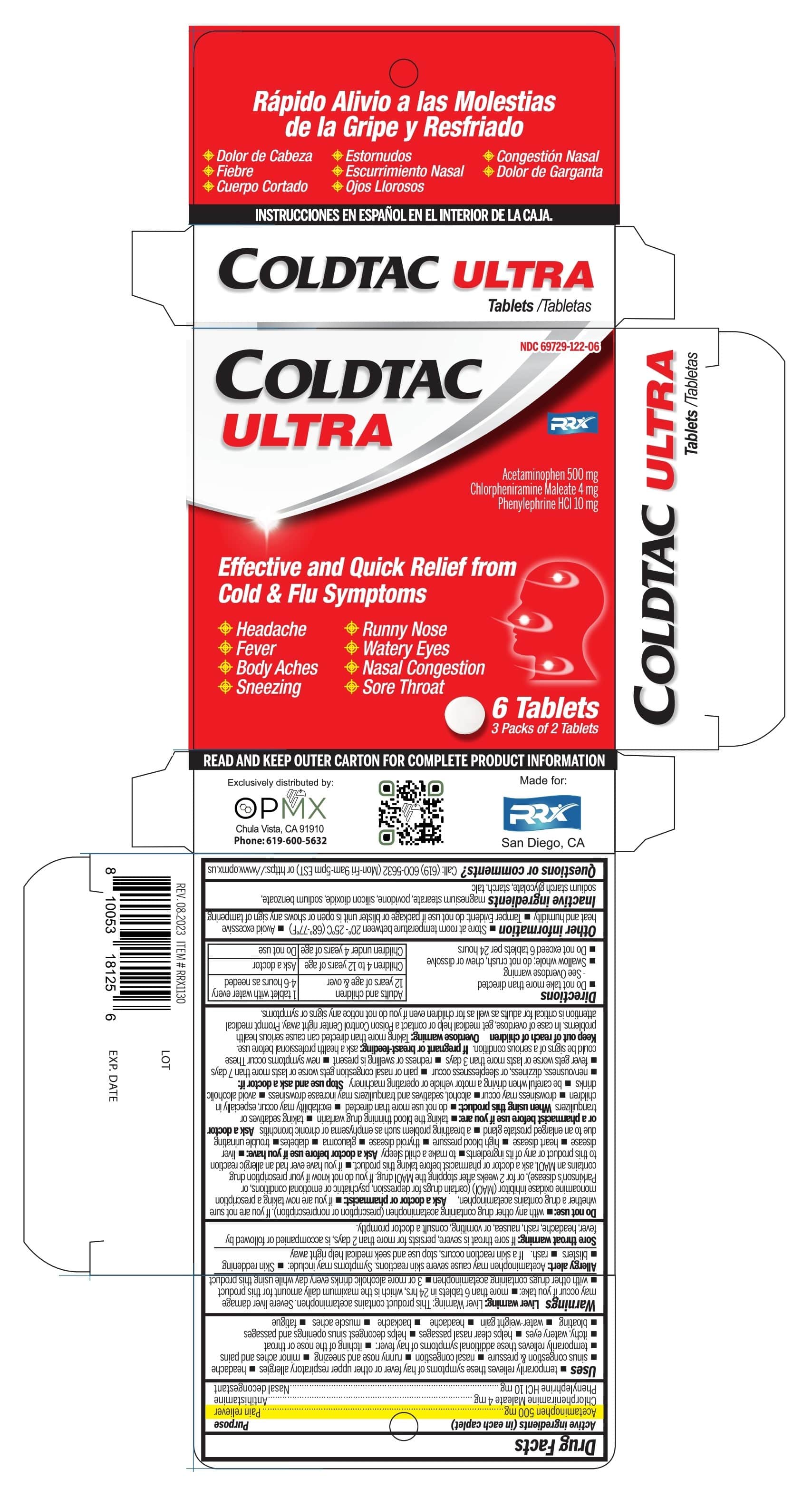
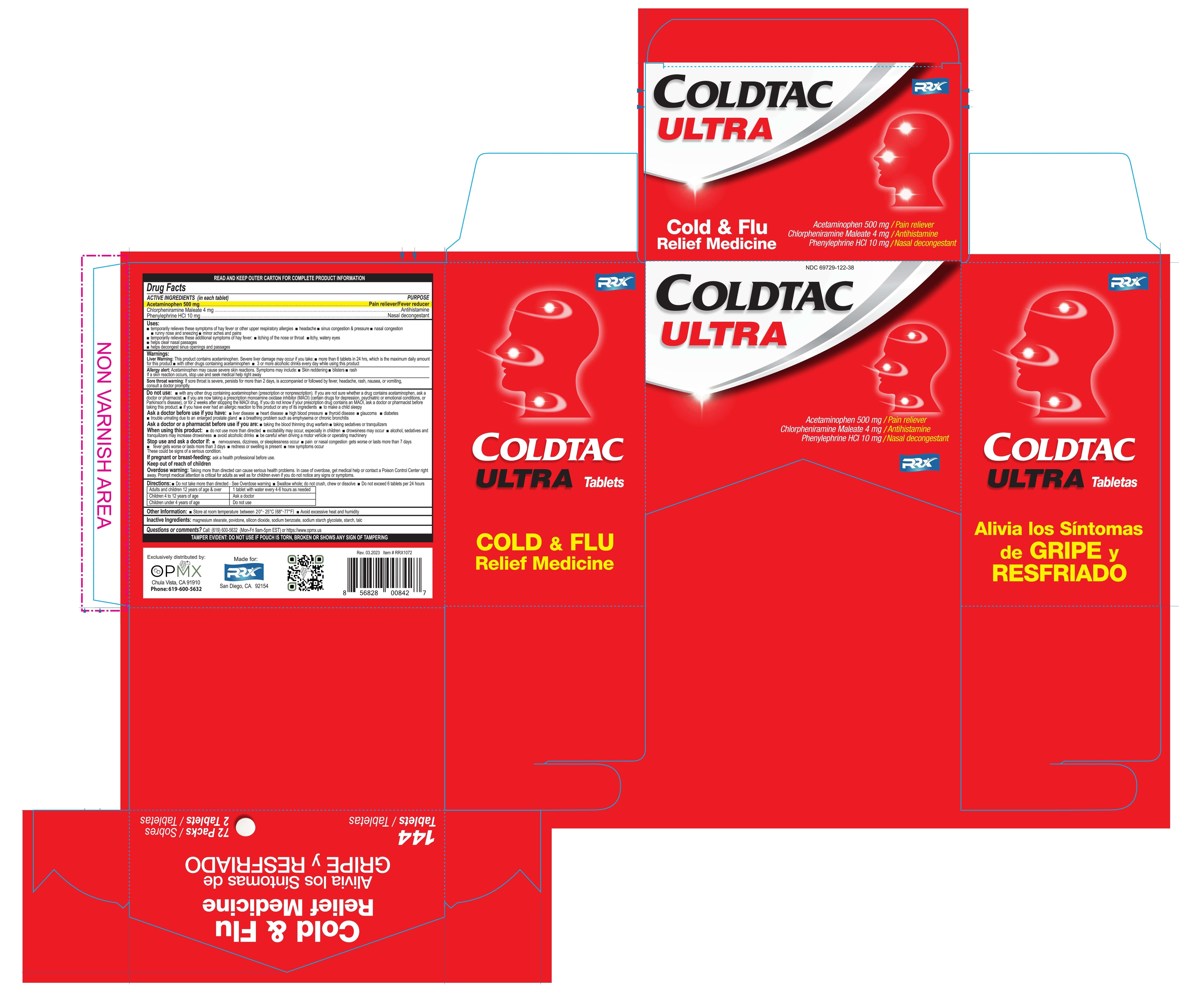
Uses
Temporarily relieves these symptoms of hay fever or other respiratory allergies:
- headache
- nasal congestion
- sinus congestion & pressure
- runny nose and sneezing
- minor aches & pain
Temporarily relieves these additional symptoms of hay fever:
- itching of the nose or throat
- itchy, watery eyes
- helps clear nasal passages
- helps decongest sinus opening and passages
Warnings
Liver warning:
This product contains acetaminophen. Severe liver damage may occur if you take :
- more than 6 tablets in 24 hours, which is the maximum daily amount for this product
- with other drugs contains acetaminophen
- 3 or more alcoholic drinks every day while using this product.
Allergy alert:
Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning:
if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult doctor promptly
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen,
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI - if you have ever had an allergic reaction to this product or any of its ingredients.
- to make a child sleepy
Ask a doctor before use if you have:
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- glaucoma
- diabetes
- trouble urinating due to enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor ot pharmacist if you are:
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product:
- do not use more than directed
- excitability may occur, especially in children
- drowsiness may occur
- alcohol, sedatives and tranquilizers may increase drowsiness
- avoid alcohol drinks
- be careful when driving a motor vehicle or operating machinery
Directions
- Do not take more than directed - See overdose warning
- Swallow whole; do not crush, chew or dissolve
- Do not exceed 6 tablets per 24 hours
| Adults and children 12 years of age & over | 1 tablet with water every 4-6 hours as needed |
| Children 4 to 12 years of age | Ask a doctor |
| Children under 4 years of age | Do not use |
Other information
- Store at room temperature between 20-25°C (68-77ºF)
- Tamper evident: Do not use if pouch is torn, broken or shows any sing of tampering