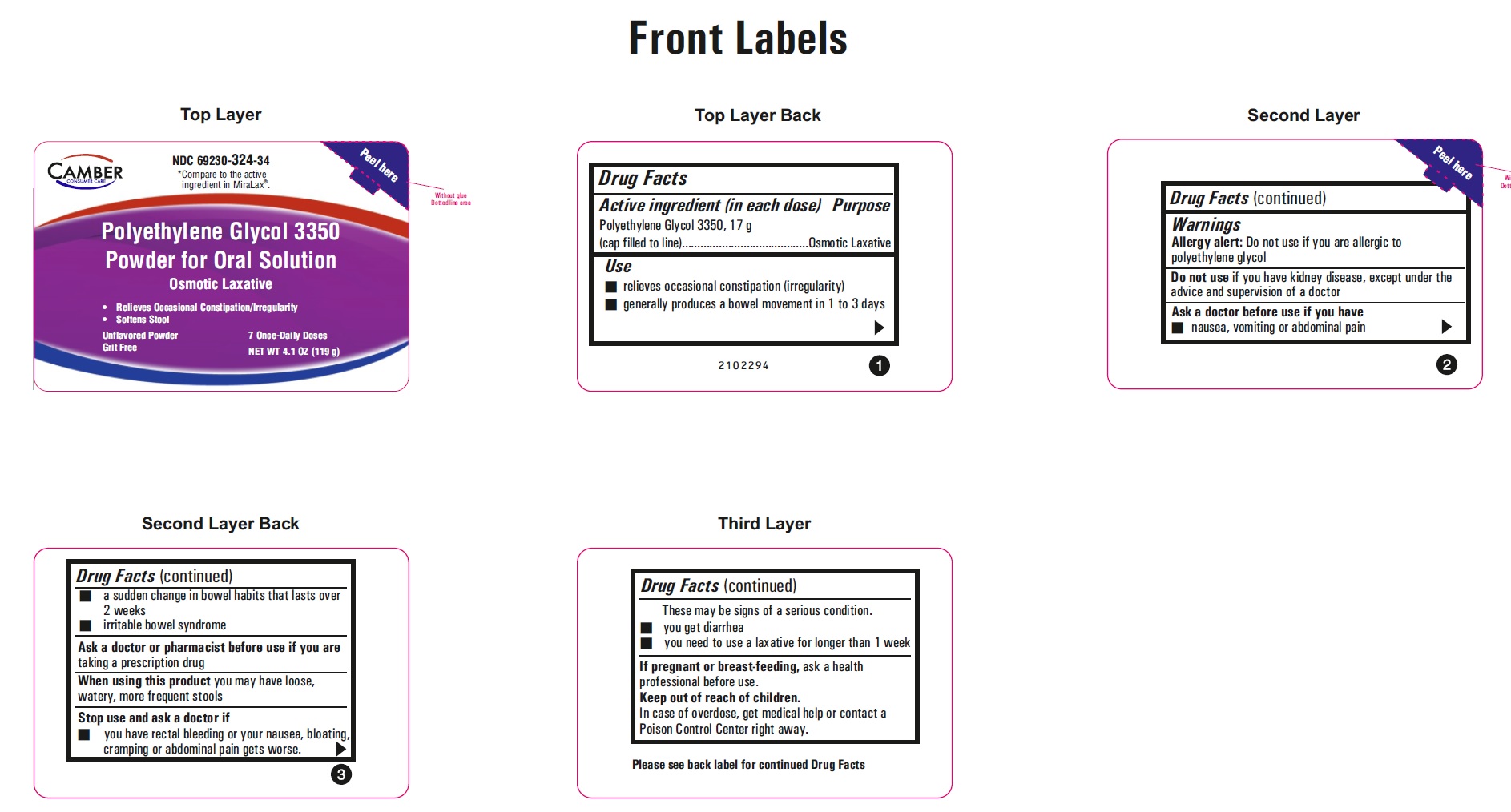
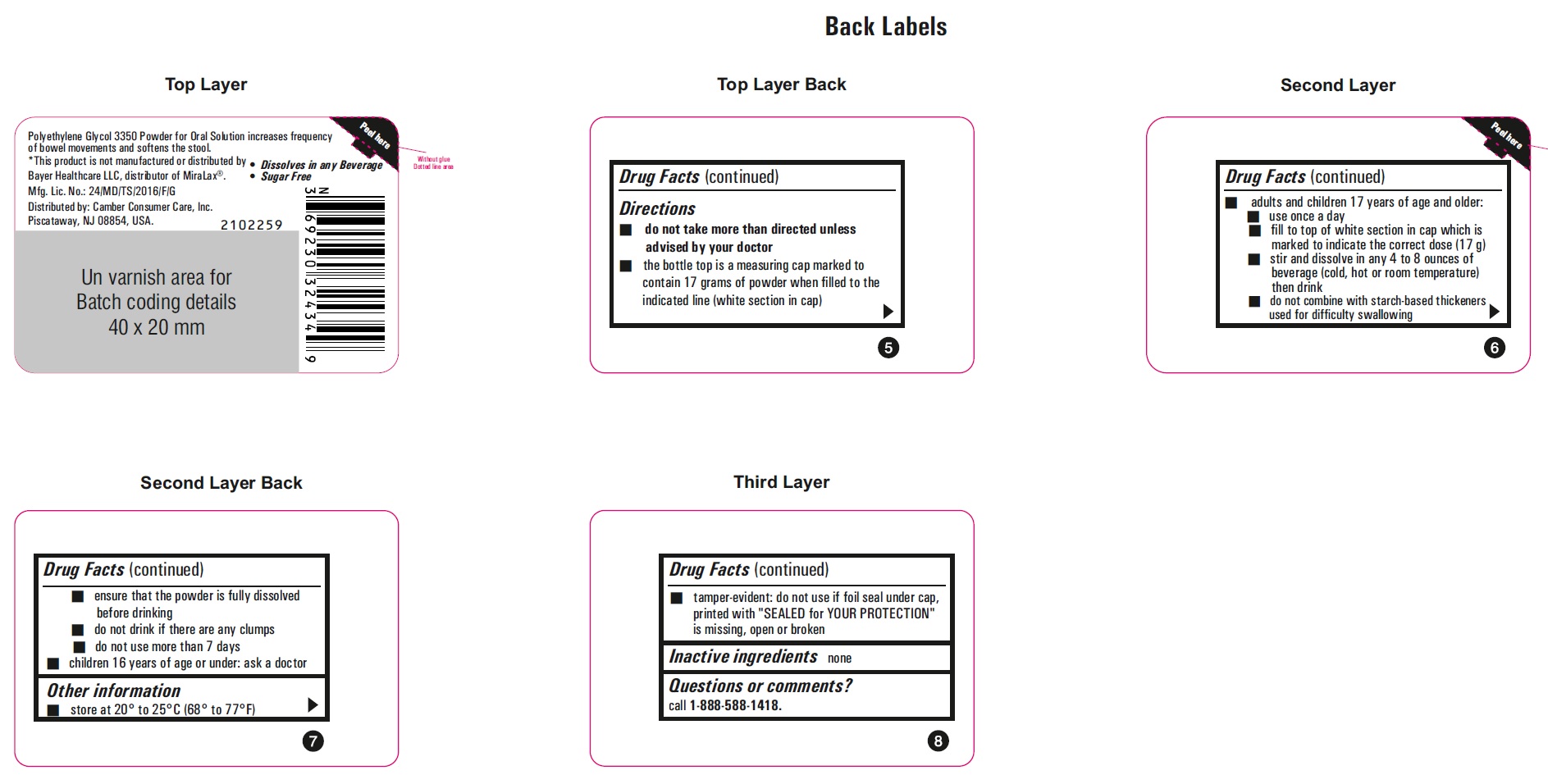
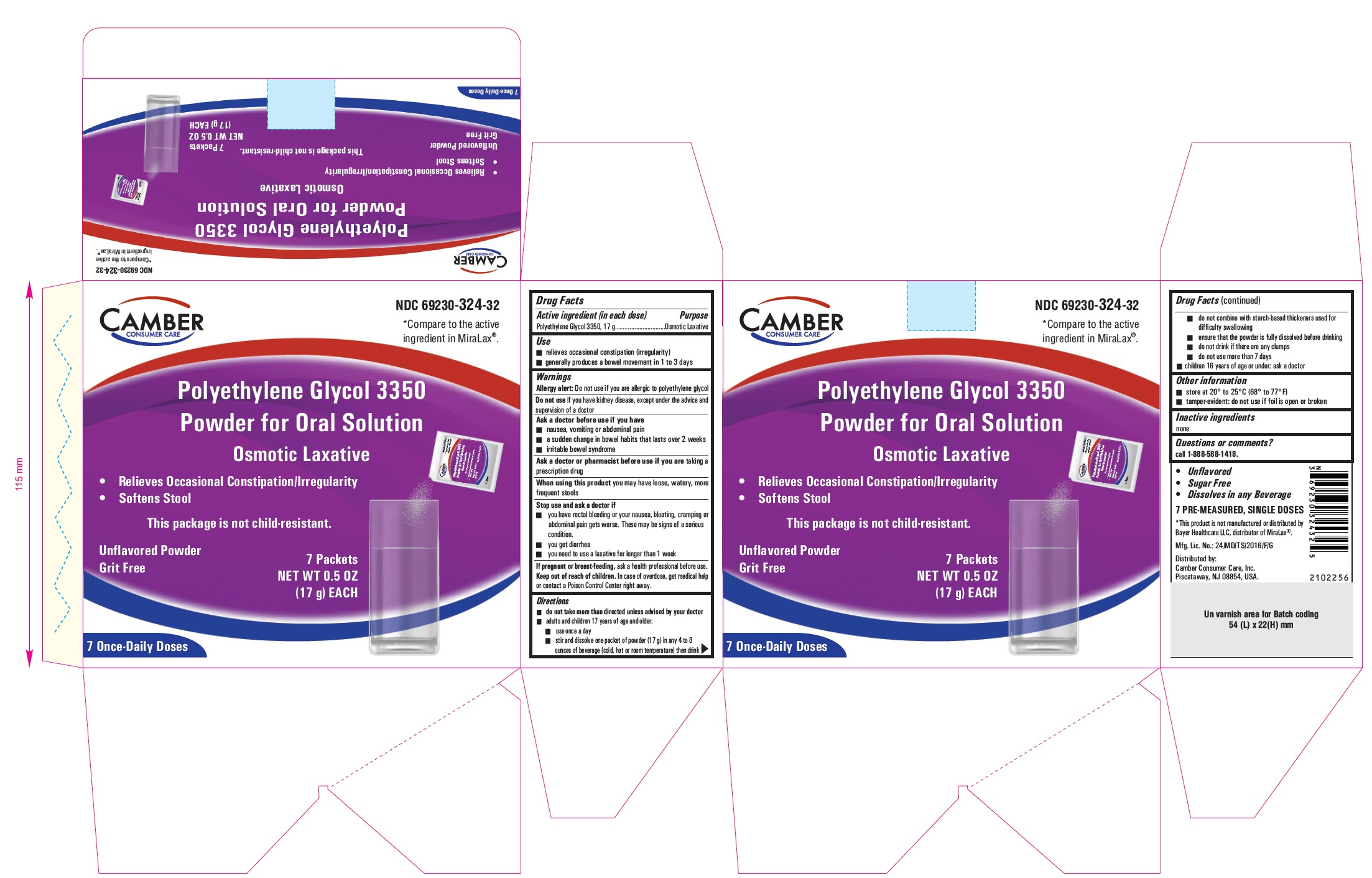
ACTIVE INGREDIENT (IN EACH DOSE)
(Bottle Only)
Polyethylene Glycol 3350, 17 g (cap filled to line)
(Packet Only)
Polyethylene Glycol 3350, 17 g
USE
• relieves occasional constipation (irregularity)
• generally produces a bowel movement in 1 to 3 days
ASK A DOCTOR BEFORE USE IF YOU HAVE
• nausea, vomiting or abdominal pain
• a sudden change in bowel habits that lasts over 2 weeks
• irritable bowel syndrome
STOP USE AND ASK A DOCTOR IF
• you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
• you get diarrhea
• you need to use a laxative for longer than 1 week
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
(Bottle Only)
•
do not take more than directed unless advised by your doctor
• the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line (white section in cap)
• adults and children 17 years of age and older:
• use once a day
• fill to top of white section in cap which is marked to indicate the correct dose (17 g)
• stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
• do not combine with starch-based thickeners used for difficult swallowing
• ensure that the powder is fully dissolved before drinking
• do not drink if there are any clumps
• do not use more than 7 days
• children 16 years of age or under: ask a doctor
(Packet Only)
• do not take more than directed unless advised by your doctor
• adults and children 17 years of age and older:
• use once a day
• stir and dissolve one packet of powder (17 g) in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
• do not combine with starch-based thickeners used for difficult swallowing
• ensure that the powder is fully dissolved before drinking
• do not drink if there are any clumps
• do not use more than 7 days
• children 16 years of age or under: ask a doctor