FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
THROMBATE III is a human antithrombin (AT) indicated in patients with hereditary antithrombin deficiency for:
- Treatment and prevention of thromboembolism
- Prevention of peri-operative and peri-partum thromboembolism
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only
2.1 Dose
- Each vial of THROMBATE III has the functional activity, in International Units (units), stated on the label of the vial. The potency assignment has been determined with a standard calibrated against a World Health Organization antithrombin reference preparation. When prepared as directed, the approximate final concentration is 50 units per milliliter.
- A guide for dosing THROMBATE III is provided in Table 1.
| Regimen (timing) | Target AT Level | Dose (Units) | Monitor AT Level |
| Loading Dose* | 120% of normal† | 120 % - baseline % x body weight (kg)
1.4% |
|
| Dose Adjustment*
(adjust as needed) | 80% to 120% of normal† | Target % - trough % x body weight (kg)
1.4% |
|
| Maintenance Dose (approximately every 24 hours, adjust as needed) | 80% to 120% of normal† | Loading Dose x 0.6 |
|
- Monitor functional plasma levels of AT. [see table above and Warnings and Precautions (5.3)], and adjust subsequent dosing based on the trough level achieved with the preceding dose until predictable peak and trough levels have been achieved, generally between 80% to 120% of normal.(1)
- Maintain plasma AT levels between 80% to 120% by administering maintenance doses of 60% of the loading dose, administered every 24 hours. Adjust the maintenance dose and interval between doses based on actual plasma AT levels achieved.
- Individualize the exact loading and maintenance dose and/or dose intervals for each patient based on the individual clinical conditions, response to therapy, and actual plasma AT levels achieved. Recovery of THROMBATE III may vary by patient. For example,
- The half-life of AT has been reported to be shortened following surgery,(2) hemorrhage or acute thrombosis, and during intravenous heparin (or low molecular weight heparin) administration.(3-6) In such conditions, monitor plasma AT levels more frequently, and administer THROMBATE III as necessary. [see Warnings and Precautions (5.3), Drug Interactions (7)]
- When an infusion of THROMBATE III is indicated for a patient with hereditary deficiency to control an acute thrombotic episode or prevent thrombosis during or following surgical or obstetrical procedures, raise the AT level to normal and maintain this level for 2 to 8 days, depending on the indication for treatment, type and extent of surgery, patient’s medical condition, past history and physician’s judgment. Base the concomitant administration of heparin in each of these situations on the medical judgment of the physician. [see Drug Interactions (7)]
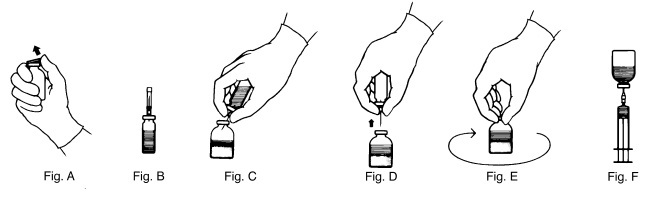
2.2 Reconstitution
- Warm THROMBATE III and Sterile Water for Injection, USP (diluent) vials to room temperature before reconstitution.
- Remove shrink band from the THROMBATE III vial. If the shrink band is absent or shows signs of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
- Remove the plastic flip top from each vial (Fig. A). Cleanse each vial stopper with an alcohol swab and allow surface to dry.
- Carefully remove the plastic sheath from the short end of the transfer needle. Insert the exposed needle into the diluent vial to the hub (Fig. B).
- Carefully grip the sheath of the other end of the transfer needle and twist to remove it.
- Invert the diluent vial and insert the attached needle into the THROMBATE III vial at a 45° angle (Fig. C). This will direct the stream of diluent against the wall of the vial and minimize foaming. The vacuum will draw the diluent into the THROMBATE III vial.*
- When diluent transfer is complete, remove the diluent vial and transfer needle (Fig. D).
- Immediately after adding the diluent, swirl the THROMBATE III vial continuously until the product is completely dissolved (Fig. E). Some foaming may occur, but attempt to avoid excessive foaming. Visually inspect the vial for particulate matter and discoloration prior to administration.
- Clean the top of the vial of reconstituted THROMBATE III again with a new alcohol swab and let surface dry.
- Attach the filter needle (from the package) to the sterile syringe. Withdraw the THROMBATE III solution into the syringe through the filter needle (Fig. F).
- Remove the filter needle from the syringe and replace with an appropriate injection or butterfly needle for administration.
- If the same patient is using more than one vial of THROMBATE III, draw the contents of multiple vials into the same syringe through the filter needles provided.
- *
- If vacuum is lost in the THROMBATE III vial during reconstitution, use a sterile syringe to remove the sterile water from the diluent vial and inject it into the THROMBATE III vial, directing the stream of fluid against the wall of the vial.
2.3 Administration
- Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Administer THROMBATE III, once reconstituted, alone without mixing with other agents or diluents.
- Administer within 3 hours following reconstitution. Do not refrigerate after reconstitution.
- Adapt the rate of administration to the response of the individual patient, but administration of the entire dose in 10 to 20 minutes is generally well tolerated.
3 DOSAGE FORMS AND STRENGTHS
THROMBATE III is a sterile lyophilized powder for reconstitution in single use vials. Each vial of THROMBATE III contains the labeled amount of antithrombin in units per vial, typically 500 units. When reconstituted with 10 mL of Sterile Water for Injection, USP, the final concentration is approximately 50 units per mL.
The potency is determined with a standard calibrated in International Units against a World Health Organization (WHO) antithrombin reference preparation.
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis, are possible. Early signs of hypersensitivity reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, hypotension, rash, nausea, vomiting, paresthesia, restlessness, wheezing and dyspnea. If hypersensitivity symptoms occur, discontinue use of the product immediately and administer appropriate emergency treatment.
5.2 Transmission of Infectious Agents
Because THROMBATE III is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent. There is also the possibility that unknown infectious agents may be present in the product. The risk that the product will transmit viruses has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and removing certain viruses during manufacture. Despite these measures, this product may still potentially transmit diseases.
Report all infections suspected by a physician possibly to have been transmitted by this product to Grifols Therapeutics LLC at 1-800-520-2807.
5.3 Monitoring: Laboratory Tests
- The effect of drugs that use antithrombin to exert their anticoagulation may be altered when THROMBATE III is added or withdrawn. Regularly perform coagulation tests suitable for the anticoagulant used (e.g., aPTT and anti-Factor Xa activity) to avoid excessive or insufficient anticoagulation. Additionally, monitor the patients for the occurrence of bleeding or thrombosis. [see Drug Interactions (7)]
- Measure functional levels of AT in plasma by amidolytic assays using chromogenic substrates or by clotting assays. Do not use immunoassays because they do not detect all hereditary AT deficiencies.
6 ADVERSE REACTIONS
In clinical studies, the most common adverse reactions (≥ 5% of subjects) were dizziness, chest discomfort, nausea, dysgeusia, and pain (cramps).
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of one drug cannot be directly compared to rates in other clinical trials of another drug and may not reflect the rates observed in clinical practice.
Two clinical trials were conducted in 33 subjects with congenital AT deficiency. The first was a prospective, open-label, dose-escalation, dose-ranging, and pharmacokinetic study in 11 asymptomatic subjects. Eight subjects received a single dose, escalated sequentially, followed by weekly dose ranging from 25 to 125 unit/kg. Five subjects (including 2 from the first part of the study) received weekly THROMBATE III for periods of up to 23 weeks in doses ranging from 125 to 225 unit/kg. The second trial was a phase III, prospective, open-label study conducted in 24 subjects for additional kinetics (n=3), the prevention of thrombosis (n=13) during high risk conditions (pregnancy, surgery), or the treatment of thrombosis (n=10). Loading doses targeted an AT plasma level of 120% and ranged from 33 to 150 unit/kg. Maintenance doses targeted a plasma AT range of 70% to 120%, which were 23 to 75 unit/kg.
Adverse reactions reported during the 2 clinical trials are listed in Table 2. Nine subjects (27%) experienced 29 adverse reactions which occurred during 17 of 389 infusions. There were no serious adverse reactions reported. The severity of adverse reactions was reported as mild or moderate, except for wound secretion and hematoma, which was severe.
|
||
| Adverse Reaction* | Number of Subjects with Adverse Reaction (%)† | Number of Adverse Reactions (% of All Infusions)‡ |
| Any adverse reaction | 9 (27) | 29 (7.5) |
| Dizziness | 4 (12) | 8 (2.1) |
| Chest discomfort | 3 (9) | 3 (0.8) |
| Nausea | 3 (9) | 3 (0.8) |
| Dysgeusia | 2 (6) | 3 (0.8) |
| Pain (cramps) | 2 (6) | 2 (0.5) |
| Chills | 1 (3) | 2 (0.5) |
| Wound secretion and hematoma | 1 (3) | 2 (0.5) |
| Vision blurred | 1 (3) | 1 (0.3) |
| Chest pain | 1 (3) | 1 (0.3) |
| Dyspnea | 1 (3) | 1 (0.3) |
| Intestinal dilatation | 1 (3) | 1 (0.3) |
| Pyrexia | 1 (3) | 1 (0.3) |
| Urticaria | 1 (3) | 1 (0.3) |
During clinical investigation of THROMBATE III, there were no reports of virus transmission. None of 12 subjects monitored for a median of 8 months (range 2–19 months) after receiving THROMBATE III became antibody positive to human immunodeficiency virus (HIV-1). None of 14 subjects monitored for ≥ 3 months demonstrated any evidence of hepatitis.
7 DRUG INTERACTIONS
The anticoagulant effect of heparin is enhanced by concurrent treatment with THROMBATE III in patients with hereditary AT deficiency. Thus, in order to avoid bleeding, the dosage of heparin (or low molecular weight heparin) may need to be reduced during treatment with THROMBATE III.
The effect of drugs that use antithrombin to exert their anticoagulation may be altered when THROMBATE III is added or withdrawn. Regularly perform coagulation tests suitable for the anticoagulant used (e.g., aPTT and anti-Factor Xa activity) and at close intervals to avoid excessive or insufficient anticoagulation. Adjust dosage of anticoagulant as necessary. Additionally, monitor the patients for the occurrence of bleeding or thrombosis.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no data with THROMBATE III use in pregnant women to inform a drug-associated risk. However, there are clinical considerations [see Clinical Considerations]. It is not known whether THROMBATE III can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. THROMBATE III should be given to a pregnant woman only if clearly needed. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Reproduction studies have been performed in rats and rabbits at doses up to four times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to THROMBATE III.
Clinical Considerations
Labor or Delivery
Suspend heparin (or low molecular weight heparin) administration and continue THROMBATE III administration during labor and delivery.
8.2 Lactation
Risk Summary
There is no information regarding the presence of THROMBATE III in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for THROMBATE III and any potential adverse effects on the breastfed infant from THROMBATE III or from the underlying maternal condition.
11 DESCRIPTION
THROMBATE III, Antithrombin III (Human), is a sterile, non-pyrogenic concentrate of human antithrombin (AT) in lyophilized powder form for reconstitution for intravenous injection. When reconstituted with Sterile Water for Injection, USP, THROMBATE III has a pH of 6.0 to 7.5 and contains 110 mEq/L to 210 mEq/L sodium, 110 mEq/L to 210 mEq/L chloride, 0.075 M to 0.125 M alanine, and not more than 0.1 unit of heparin per 1 unit of AT. THROMBATE III contains no preservative.
THROMBATE III is prepared from pooled units of human plasma from normal donors. The capacity of the THROMBATE III manufacturing process to remove and/or inactivate enveloped and non-enveloped viruses has been validated by laboratory spiking studies on a scaled down process model using a wide range of viruses with diverse physicochemical properties. There are two dedicated virus inactivation/removal steps included in the THROMBATE III manufacturing process: a heat treatment step at 60°C ± 0.5°C for not less than 10 hours for virus inactivation and a nanofiltration step for effective removal of viruses as small as 18 nm.
The THROMBATE III manufacturing process was also investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the variant Creutzfeldt-Jakob disease (vCJD) and Creutzfeldt-Jakob disease (CJD) agents. An individual production step in the THROMBATE III manufacturing process has been shown to decrease TSE infectivity of that experimental model agent. The TSE reduction step is the Effluent I to Effluent II + III fractionation step (6.0 log10). These studies provide reasonable assurance that low levels of vCJD/CJD agent infectivity, if present in the starting material, would be removed.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Antithrombin, an alpha2-glycoprotein of molecular weight 58,000, is normally present in human plasma at a concentration of approximately 12.5 mg/dL and is the major plasma inhibitor of thrombin. Inactivation of thrombin by AT occurs by formation of a covalent bond resulting in an inactive 1:1 stoichiometric complex between the two, involving an interaction of the active serine of thrombin and an arginine reactive site on AT. AT is also capable of inactivating other components of the coagulation cascade including factors IXa, Xa, XIa, and XIIa, as well as plasmin. The neutralization rate of serine proteases by AT proceeds slowly in the absence of heparin, but is greatly accelerated in the presence of heparin. As the therapeutic antithrombotic effect of heparin is mediated by AT, heparin in vivo is ineffective in the absence or near absence of AT.
After administration, THROMBATE III temporarily replaces the missing AT in patients with hereditary antithrombin deficiency.
12.3 Pharmacokinetics
In a clinical trial of THROMBATE III conducted in asymptomatic subjects with hereditary deficiency of AT, 8 subjects were administered a single dose of THROMBATE III at doses ranging from 25 units/kg to 125 units/kg. Pharmacokinetic parameters were determined using immunologic and functional AT assays (Table 3).
| Immunological Assay | Functional Assay | |
|
||
| AT recovery, % / unit / kg | 1.6 ± 0.1* | 1.4 ± 0.1 |
| 50% disappearance time, hr | 17.4 ± 3.9 | 22.3 ± 8.6 |
| t½, day | 2.5 ± 1.5 | 3.8 ± 1.8 |
14 CLINICAL STUDIES
In a prospective, open-label clinical trial, 21 subjects were administered THROMBATE III for 16 prophylaxis events (n=13 subjects) and 10 for treatment of thrombosis (n=10 subjects) with 2 subjects receiving THROMBATE III for both prophylaxis and treatment of thrombosis. None of the 13 subjects with hereditary AT deficiency and histories of thromboembolism treated prophylactically on 16 separate occasions with THROMBATE III for high thrombotic risk situations (11 surgical procedures, 5 pregnancies and/or deliveries) developed a thrombotic complication. Heparin was administered in 3 of the 11 surgical procedures. Two of the pregnant subjects received LMW heparin prophylactically during the first trimester, but which was unable to maintain anti-coagulation with increasing dosages. [see Drug Interactions (7)] They experienced a thrombosis, which subsequently resolved with the addition of THROMBATE III, and were therefore administered THROMBATE III and LMW heparin prophylaxis weekly during the second and third trimesters, and during labor and delivery. These two subjects did not experience a new thrombosis.
Ten subjects with hereditary AT deficiency were treated with THROMBATE III as well as heparin (n=9) for major thrombotic or thromboembolic complications, including 4 subjects with thrombosis during the first trimester of pregnancy. Nine subjects recovered with no additional thromboses or extension of existing thrombosis. The tenth subject died due to complications from the original pulmonary embolism with infarction which preceded treatment with THROMBATE III.
15 REFERENCES
- Schwartz RS, Bauer KA, Rosenberg RD, et al. Clinical experience with antithrombin III concentrate in treatment of congenital and acquired deficiency of antithrombin. Am J Med. 1989;87 (Suppl 3B):53S-60S.
- Mannucci PM, Boyer C, Wolf M, et al. Treatment of congenital antithrombin III deficiency with concentrates. Br J Haematol. 1982;50(3):531-5.
- Collen D, Schetz J, de Cock F, et al. Metabolism of antithrombin III (heparin cofactor) in man: effects of venous thrombosis and of heparin administration. Eur J Clin Invest. 1977;7(1):27-35.
- Marciniak E, Gockerman JP. Heparin-induced decrease in circulating antithrombin-III. Lancet. 1977;2(8038):581-4.
- O’Brien JR, Etherington MD. Effect of heparin and warfarin on antithrombin III. Lancet. 1977;2(8050):1232.
- Kakkar VV, Bentley PG, Scully MF, et al. Antithrombin III and heparin. Lancet. 1980;1(8159):103-4.
16 HOW SUPPLIED/STORAGE AND HANDLING
THROMBATE III is supplied in a kit containing one single use vial of THROMBATE III lyophilized powder for reconstitution, one vial of Sterile Water for Injection, USP, one sterile double-ended transfer needle, and one sterile filter needle. The total activity of AT in International Units is stated on the label of the THROMBATE III vial.
Components of the packaging do not contain natural rubber latex.
| NDC Number Carton (Kit) | Approximate Antithrombin Potency | Diluent |
| 13533-603-20, 13533-602-50 or 13533-606-12 | 500 units | 10 mL |
- Store THROMBATE III at temperatures not to exceed 25°C (77°F).
- Avoid freezing as breakage of the diluent vial might occur.
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Inform patients that allergic-type hypersensitivity reactions are possible and instruct them to inform their physicians about any past or present known hypersensitivity to human plasma proteins prior to treatment with THROMBATE III. Inform patients of the early signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis and to notify their health care provider immediately if these events develop. [see Warnings and Precautions (5.1)]
Transmission of Infectious Disease
Inform patients that THROMBATE III is made from human plasma and may carry a risk of transmitting infectious agents that can cause disease (e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent). Inform patients that this risk has been reduced by screening plasma donors for prior exposure to certain infectious agents, by testing the donated plasma for markers of certain current infections, and by inactivating and/or removing pathogens during manufacturing. [see Warnings and Precautions (5.2)]
Manufactured by:
GRIFOLS
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3056479
PACKAGE LABEL
NDC 13533-605-21
500 IU / 10 mL
Antithrombin III
(Human)
Thrombate III®
Lyophilized Powder
Rx only
For Intravenous
Administration Only
Heat-Treated Nanofiltered
The patient and physician should discuss
the risks and benefits of this product.
No Preservative
Sterile — Nonpyrogenic
Reconstitute with 10 mL Sterile Water for
Injection, USP.
Store at temperatures not to exceed 25°C
(77°F). Do not freeze.
Dosage and Administration: Read package
insert.
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3060449
Lot
Exp.
IU

NDC 13533-602-50 500 IU / 10 mL
Antithrombin III (Human)
Thrombate III®
Lyophilized Powder
Rx only
For Intravenous Administration Only
GRIFOLS
Heat-Treated
Nanofiltered
Contents:
One vial of THROMBATE III
10 mL Sterile Water for Injection,
USP
One sterile filter needle
One sterile double-ended transfer
needle
No Preservative
Sterile — Nonpyrogenic
GRIFOLS
The patient and physician should discuss the risks and benefits of
this product.
This product when reconstituted with 10 mL Sterile Water for
Injection, USP, contains 110-210 mEq/L sodium, 110-210 mEq/L
chloride, 0.075-0.125 M alanine, and not more than
0.1 IU heparin/IU ATIII.
Administer within 3 hours after reconstitution.
Dosage and Administration: Read enclosed package insert.
Store at temperatures not to exceed 25°C (77°F). Do not
freeze.
If the shrink band is absent or shows any sign of tampering, do not
use the product and notify Grifols Therapeutics LLC immediately.
Not Returnable for Credit or Exchange
GRIFOLS
This product is prepared from large
pools of human plasma which may
contain infectious agents.
See package insert Warnings.
Grifols Therapeutics LLC
Research Triangle Park,
NC 27709 USA
U.S. License No. 1871
GRIFOLS
Open other end
Carton: 3060457
GTIN XXXXXXXXXXXXXX
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN 4001XXXXXXXXXX
IU XXX
NDC 13533-602-50 500 IU / 10 mL
Antithrombin III (Human)
Thrombate III®
NDC 13533-200-10
Sterile Water for Injection, USP
10 mL
Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other
substance has been added. Do not use for intravascular
injection without making approximately isotonic by
addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S. A., Parets del Vallès, Barcelona, 08150 Spain
Mfd for: Grifols Therapeutics LLC, Research Triangle Park, NC, 27709 USA
3051810
Lot / Exp.

NDC 76297-002-12
Sterile Water for Injection, USP
10 mL
Rx Only
For reconstitution of accompanying product
Single-Dose Container, Nonpyrogenic
Do not use unless clear. No antimicrobial agent or other
substance has been added. Do not use for intravascular
injection without making approximately isotonic by
addition of suitable solute. Discard unused portion.
Mfd by: Laboratorios Grifols, S. A. Parets del Vallès,
Barcelona 08150 Spain
3057423
Lot
EXP