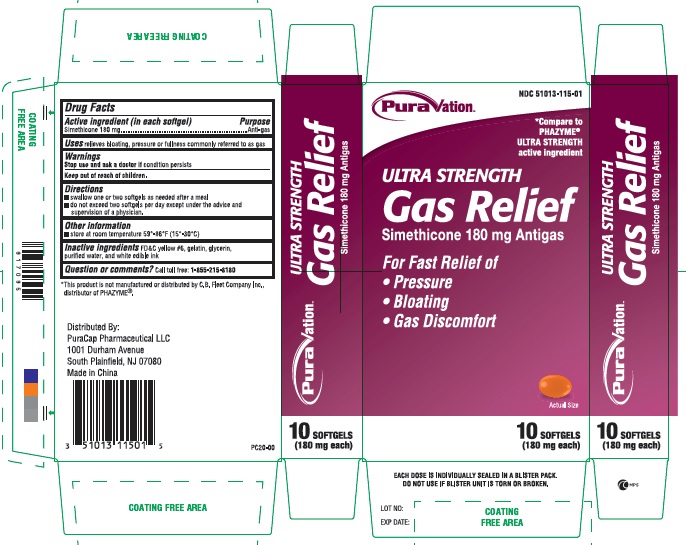
GAS RELIEF- simethicone capsule, liquid filled
PuraCap Pharmaceutical LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (in each softgel)
Simethicone 180 mg
Uses
relieves bloating, pressure or fullness commonly referred to as gas
Warnings
Stop use and ask a doctorif condition persists
Keep out of reach of children.
Directions
- swallow one or two softgels as needed after a meal
-
do not exceed two softgels per day except under the advice and supervision of a physician.
Other information
-
store at room temperature 59º - 86ºF (15º - 30ºC)
Inactive ingredients
FD&C yellow#6, gelatin, glycerin, purified water and white edible ink
Questions or comments?
Call toll free: 1-855-215-8180
PRINCIPAL DISPLAY PANEL
ULTRA STRENGTH Gas Relief
Simethicone 180 mg Antigas
NDC 51013-115-01
*Compare to the active ingredient in PHAZYME® Ultra Strength