FULL PRESCRIBING INFORMATION
WARNING: THROMBOSIS
● Serious arterial and venous thrombotic events may occur following administration of SEVENFACT®. [See Warnings and Precautions (5.1)]
● Discuss the risks and explain the signs and symptoms of thrombotic and thromboembolic events to patients who will receive SEVENFACT®.
● Monitor patients for signs or symptoms of activation of the coagulation system and for thrombosis.
1 INDICATIONS AND USAGE
SEVENFACT [coagulation factor VIIa (recombinant)-jncw] is indicated for the treatment and control of bleeding episodes occurring in adults and adolescents (12 years of age and older) with hemophilia A or B with inhibitors.
Limitation of Use: SEVENFACT is not indicated for the treatment of patients with congenital Factor VII deficiency.
2 DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
2.1 Dose
- Dose and duration of treatment depend on the location and severity of the bleeding, need for urgent hemostasis, frequency of administration, and known patient responsiveness to FVIIa-containing bypassing agents during prior bleeding events. Treatment with SEVENFACT should be initiated as soon as a bleeding event occurs.
- The dose, frequency, and duration of SEVENFACT therapy should be based on the patient’s clinical response and hemostasis evaluation.
- The use of laboratory assessment(s) of coagulation (PT/INR, aPTT, FVII:C) does not necessarily correlate with or predict the hemostatic effectiveness of SEVENFACT.
- Maximum tolerated doses have not been determined for SEVENFACT, and cumulative daily doses greater than 900 mcg/kg, which may be associated with greater risk of thromboembolic complications, have not been studied.
- Dose adjustment may be required if the patient has received other procoagulant therapies prior to treatment with SEVENFACT.
Based on the clinical trial program for SEVENFACT, the recommended initial dose should be adjusted based on the criteria provided in Table 1.
| Type of Bleeding | Dosing Regimen Recommendation | Duration of Therapy |
| Mild and Moderate
Joint, superficial muscle, soft tissue and mucous membranes. | 75 mcg/kg repeated every 3 hours until hemostasis is achieved
or Initial dose of 225 mcg/kg. If hemostasis is not achieved within 9 hours, additional 75 mcg/kg doses may be administered every 3 hours as needed to achieve hemostasis. Consider alternative treatments if successful control of bleeding does not occur within 24 hours of the first administration of SEVENFACT. Consider the following factors when choosing the initial dose of SEVENFACT:
| Continue therapy to support healing and prevent recurrent hemorrhage after hemostasis to maintain the hemostatic plug. The site and severity of bleeding should determine therapy duration. |
| Severe
Life or limb threatening hemorrhage, iliopsoas and deep muscle with neurovascular injury, retroperitoneum, intracranial, or gastrointestinal. Patients should seek immediate medical care if signs or symptoms of severe bleeding occur in the home setting. | 225 mcg/kg initially, followed if necessary 6 hours later with 75 mcg/kg every 2 hours until hemostasis is achieved.
Subsequent Dosing: After achieving hemostasis, base the decision for dosing on clinical assessment and the type of bleeding. Consider the risk of thrombosis with subsequent dosing after achieving hemostatic efficacy. | Continue therapy to support healing and prevent recurrent hemorrhage. The site and severity of bleeding and the use of other procoagulant therapies should determine therapy duration. |
2.2 Reconstitution
- Follow the procedures below for reconstitution of SEVENFACT.
- Calculate the amount of SEVENFACT required and select the appropriate SEVENFACT packages containing the matching pre-filled syringe of sterile Water for Injection, and the vial adapters.
- Reconstitute each vial with the pre-filled syringe provided with each vial of SEVENFACT.
Overview of SEVENFACT Package:
Figure 1 Vial with SEVENFACT lyophilized powder
Lyophilized Powder Drug Vial
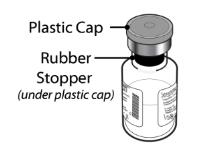
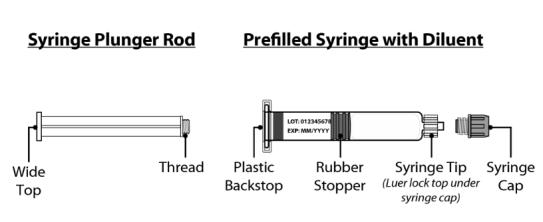
Figure 2 Syringe plunger rod and pre-filled syringe with Water for Injection diluent
Syringe Plunger Rod Pre-filled syringe with Diluent
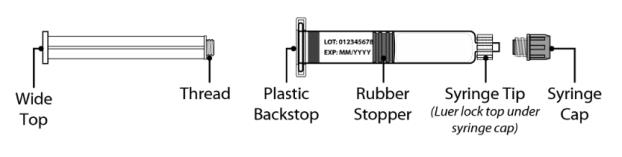
Figure 3 SEVENFACT 1 mg vial adapter and SEVENFACT 5 mg vial adapter
Vial Adapters* and Packaging
1 mg vial adapter 5 mg vial adapter
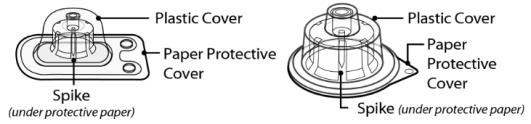
*Note: Each SEVENFACT kit will contain only one vial adapter.
The instructions below serve as a general guideline for reconstitution of SEVENFACT.
Reconstitution:
- Based on the prescribed dose, take out the number of SEVENFACT kits (each kit containing one vial of SEVENFACT powder and one pre-filled Water for Injection diluent syringe with one vial adapter for needleless reconstitution), an infusion set (not supplied in the kit) and an alcohol swab (not supplied in the kit). Check the expiration date on the side of the box(es) for the SEVENFACT kit(s).
- Always use aseptic technique. Wash your hands with soap and water and dry them using a clean towel or air dry.
- Take out the contents of one kit and one alcohol swab. Place items on a clean surface.
- Inspect all contents of the kit. Make sure each vial has a matching colored syringe.
- Bring SEVENFACT (lyophilized powder) and the specified pre-filled syringe (diluent) to room temperature. The specified volume of diluent corresponding to the amount of SEVENFACT is as follows:
1 mg (1000 micrograms) vial + 1.1 mL Water for Injection diluent in pre-filled syringe
5 mg (5000 micrograms) vial + 5.2 mL Water for Injection diluent in pre-filled syringe
- Remove the plastic cap from the SEVENFACT vials to expose the central portion of the rubber stopper. Cleanse the rubber stoppers with an alcohol swab and allow to dry prior to use.
- Peel back the protective paper from the vial adapter. Do not remove the vial adapter from the package.
- Place the SEVENFACT vial on a flat surface. While holding the vial adapter package, place the vial adapter over the SEVENFACT vial and press down firmly on the package until the vial adapter spike breaks through the rubber stopper.
- Lightly squeeze the plastic cover and lift up to remove it from the vial adapter. Note: the 5 mg vial adapter may not sit flat against the vial, but it is fully functional.
- Remove the syringe cap from the pre-filled syringe by holding the syringe body with one hand to unscrew the syringe cap (turn to the left).
- While holding the edges of the vial adapter, screw on the pre-filled syringe (turn to the right) a few turns until it starts to tighten.
- Insert the plunger rod into the syringe, then screw a few turns (turn to the right) so that the plunger rod is attached to the gray rubber stopper in the syringe.
- Push the plunger rod to slowly inject all the diluent into the vial. Keep the plunger rod pressed down and swirl the vial gently until the powder is dissolved.
- The reconstituted solution is clear to slightly opaque. All powder must be mixed with no particles floating in the liquid.
- Without withdrawing any drug back into the syringe, unscrew the syringe from the vial adapter (turn to the left) until it is completely detached.
- Withdraw the liquid drug from the vial(s), using an infusion syringe provided by the pharmacy; the syringe should be large enough to hold the prescribed dose.
- The reconstituted solution should be stored in the vial at room temperature, but can be stored between 36oF to 86oF (2oC to 30oC) for up to 4 hours after reconstitution. After reconstitution with the specified volume of diluent, each vial contains approximately 1 mg per mL SEVENFACT (1000 micrograms per mL).
2.3 Administration
For Intravenous Use Only.
- Visually inspect the reconstituted solution for particulate matter and discoloration prior to administration. Do not use if particulate matter or discoloration is observed.
- Do not freeze reconstituted solution or store it in a syringe.
- SEVENFACT must be infused within 4 hours after reconstitution.
- SEVENFACT should be infused over 2 minutes or less as a bolus intravenous infusion.
- Do not mix with other infusion solutions.
- Any unused solution should be discarded 4 hours after reconstitution.
3 DOSAGE FORMS AND STRENGTHS
SEVENFACT is a white to off-white lyophilized powder for reconstitution in a colorless solution for injection. It is supplied in single-dose vial sizes containing 1 mg or 5 mg of coagulation factor VIIa (recombinant)-jncw.
The diluent for reconstitution of SEVENFACT is supplied in single-dose prefilled glass syringes containing 1.1 mL or 5.2 mL sterile Water for Injection. It is a clear colorless solution.
After reconstitution with the appropriate volume of Water for Injection diluent, each mL of SEVENFACT contains 1 mg per mL of coagulation factor VIIa (recombinant)-jncw (1,000 micrograms per mL).
4 CONTRAINDICATIONS
SEVENFACT is contraindicated in
- known allergy to rabbits or rabbit proteins. Exposure to SEVENFACT in these patients can result in severe hypersensitivity reaction.
- patients with severe hypersensitivity reaction to SEVENFACT or any of its components. Exposure to SEVENFACT in these patients can result in severe hypersensitivity reaction.
5 WARNINGS AND PRECAUTIONS
5.1 Thrombosis
- There is limited information about the safety of SEVENFACT in patients with a history of arterial or venous thromboembolic disease, because such patients were excluded from SEVENFACT trials. Serious arterial and venous thrombotic reactions can occur with SEVENFACT. Such reactions have been reported in clinical trials and post-marketing surveillance with a similar class of products. Neither arterial nor venous thrombotic events have been reported in SEVENFACT clinical trials.
- The following patients may have increased risk of thromboembolic events with use of SEVENFACT:
- History of congenital or acquired hemophilia receiving concomitant treatment with aPCC/PCC (activated or non-activated prothrombin complex) or other hemostatic agents
- History of atherosclerotic disease, coronary artery disease, cerebrovascular disease, crush injury, septicemia, or thromboembolism.
- Monitor patients who receive SEVENFACT for the development of signs and symptoms of activation of the coagulation system or thrombosis. When there is laboratory confirmation of intravascular coagulation or presence of clinical thrombosis, reduce the dose of SEVENFACT or stop treatment, depending on the patient’s condition.
5.2 Hypersensitivity Reactions
- No hypersensitivity reactions have been reported in SEVENFACT trials; however, hypersensitivity reactions, including anaphylaxis, can occur with SEVENFACT. Symptoms may include hives, itching, rash, difficulty breathing, swelling around the mouth and throat, tightness of the chest, wheezing, dizziness or fainting, and low blood pressure. In the event of hypersensitivity reactions, patients should discontinue treatment and seek immediate medical attention.
- Patients with known IgE-based hypersensitivity to casein may be at higher risk of hypersensitivity reactions. Should signs or symptoms of hypersensitivity occur, treatment should be discontinued. Subsequent treatment with SEVENFACT should be based on a thorough assessment of the risks and benefits.
5.3 Neutralizing Antibodies
- In the studies performed, no patients tested positive for neutralizing antibodies. Nevertheless, neutralizing antibodies may occur with the use of SEVENFACT. If treatment with SEVENFACT does not result in adequate hemostasis, then suspect development of neutralizing antibody as the possible cause and perform testing as clinically indicated.
- Neutralizing antibodies to other Factor VIIa-containing products have been observed in congenital Factor VII-deficient patients. SEVENFACT has not been studied in this patient population. [See limitation of use statement under Indications and Usage (1)].
6 ADVERSE REACTIONS
The most common adverse reactions (incidence ≥1%) reported in clinical trials for SEVENFACT were headache, dizziness, infusion-site discomfort, infusion-site hematoma, infusion-related reaction, and fever.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two studies of SEVENFACT in patients with Hemophilia A or B with or without inhibitors, 42 subjects (27 subjects in Study 1 and 15 subjects in Study 2) have been exposed to SEVENFACT.
Study 1: The safety of SEVENFACT has been evaluated in a safety and efficacy study of 27 subjects with Hemophilia A or B with inhibitors, which included treatment of 468 bleeding episodes. As described in Table 2, a total of seven adverse reactions were observed in two subjects (7.4%) treated with SEVENFACT. One episode of fever occurred in a 12-year-old subject, lasted two days, and was managed symptomatically.
Study 2: In a study with 15 subjects evaluating the safety and pharmacokinetics of three escalating doses of SEVENFACT (25 mcg/kg, 75 mcg/kg and 225 mcg/kg), a total of three (20%) subjects experienced four adverse reactions (Table 2).
One subject developed an infusion reaction immediately following the infusion of the first dose of 75 mcg/kg; the reaction lasted 45 minutes. Signs and symptoms included flushing, chest tightness, shakiness, transient tachycardia, and mild hypotension. Symptoms resolved without any intervention and did not recur with subsequent administration at 225 mcg/kg dose.
Adverse reactions reported in the two clinical studies are shown in Table 2.
| Preferred Terms | Number of Adverse Reactions in Study 2
(N=15) | Number of Adverse Reactions in Study 1
(N=27) |
| Infusion site discomfort | - | 4 |
| Infusion site hematoma | - | 2 |
| Dizziness | 2 | - |
| Headache | 1 | - |
| Body temperature increased | - | 1 |
| Infusion related reaction | 1 | - |
6.2 Immunogenicity
In Study 1, two out of 27 subjects had a positive screening assay for anti-SEVENFACT antibody at baseline, prior to exposure to SEVENFACT, and at follow-up visits. One of these two subjects had a transient SEVENFACT antibody with an additional confirmatory test for anti-SEVENFACT antibody, which was confirmed as non-neutralizing.
In Study 2, five of 15 subjects tested positive for anti-SEVENFACT antibody using a screening assay. The confirmatory assay was negative for all subjects at all time points.
No subject developed anti-rabbit milk protein antibodies during treatment with SEVENFACT.
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibodies is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to SEVENFACT with the incidence of antibodies to other products may be misleading.
7 DRUG INTERACTIONS
Clinical experience with pharmacologic use of FVIIa-containing products indicates an elevated risk of serious thrombotic events when used simultaneously with activated prothrombin complex concentrates.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies using SEVENFACT in pregnant women to determine whether there is a drug-associated risk. Animal studies evaluating the embryo-fetal teratogenic potential of SEVENFACT have not been conducted. It is unknown whether SEVENFACT can cause fetal harm when administered to a pregnant woman or can affect fertility.
In the U.S. general population, the estimated background risks of major birth defect and miscarriage in clinically recognized pregnancies are 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of SEVENFACT in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for SEVENFACT and any potential adverse effects on the breastfed infant from SEVENFACT or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Risk Summary
In Study 1, male patients cautioned to avoid sexual activity without condoms received SEVENFACT for the treatment of bleeding episodes. No pregnancies from sexual partners were reported. The relative benefits of SEVENFACT should be weighed against any potential risk arising from exposure in sexually active patients.
All clinical studies of SEVENFACT were performed on males, as males are predominantly affected with the congenital form of hemophilia. No adverse effects on the mating index, fertility, or conception rate were observed following administration of SEVENFACT at dose levels up to 13-fold higher than the highest recommended human dose in healthy male rats prior to and during cohabitation with healthy untreated female rats [See Carcinogenesis, Mutagenesis, Impairment of Fertility (13.1)].
8.4 Pediatric Use
The safety and effectiveness of SEVENFACT have been established for pediatric patients ≥ 12 years of age for the treatment and control of bleeding episodes. Limited clinical data for SEVENFACT in adolescents (≥12 to <18 years) were collected in an adult and adolescent study (Study 1). A total of 5 subjects were dosed with SEVENFACT. These 5 subjects were treated for a total of 79 bleeding episodes (all mild or moderate) that occurred while subjects were still under 18 years of age. Hemostatic efficacy in this subgroup (n=5) was comparable to efficacy observed in the overall population [See Clinical Studies (14)].
The safety and effectiveness of SEVENFACT for the treatment and control of bleeding episodes have not been established in children < 12 years of age. Effectiveness was not demonstrated in a trial of 25 pediatric patients 6 months to < 12 years of age. The safety and effectiveness of SEVENFACT in infants less than 6 months of age have not been evaluated.
8.5 Geriatric Use
Safety and effectiveness of SEVENFACT in patients >65 years of age have not been evaluated in clinical trials. The presence of age-related comorbidities and the attendant risks associated with thrombotic and thromboembolic events should be considered when administering SEVENFACT to patients older than 50 years of age.
10 OVERDOSAGE
There have been no reports of overdosage with SEVENFACT. Doses greater than 900 mcg/kg/day have not been studied. Doses greater than 900 mcg/kg per 24 hours may be associated with an increased risk of thromboembolic events.
11 DESCRIPTION
SEVENFACT is a sterile, white to off-white lyophilized powder in a single-use vial containing either 1 mg or 5 mg of coagulation factor VIIa (recombinant)-jncw as the active ingredient. SEVENFACT is to be reconstituted with Sterile Water for Injection in a pre-filled syringe supplied with the product. The reconstituted product is a clear to slightly opaque solution of coagulation factor VIIa (recombinant)-jncw at a concentration of 1 mg of protein per mL with a pH of approximately 6.0. SEVENFACT is formulated with arginine, isoleucine, citrate, glycine, lysine and polysorbate 80. It does not contain any antimicrobial preservatives nor human or bovine plasma-derived proteins.
The active ingredient in SEVENFACT, activated coagulation Factor VII, is a glycoprotein of 406 amino acids with a molecular weight of approximately 50 kilodaltons. The amino acid sequence of activated coagulation Factor VII is identical to that of human plasma-derived Factor VIIa. It is >99% pure with a nominal specific activity of 45,000 IU/mg of protein when tested against the World Health Organization international standard for human Factor VIIa activity.
SEVENFACT is produced by recombinant DNA technology using genetically engineered rabbits into which the DNA coding sequence for human Factor VII has been introduced. Human Factor VII is expressed in the rabbit mammary gland and secreted into the milk. During purification and processing, Factor VII is enzymatically converted to activated Factor VII. The manufacturing process of SEVENFACT includes specific steps to reduce impurities. SEVENFACT may contain trace amounts of rabbit proteins. The purification process also includes steps that are validated to inactivate or remove viruses, such as Xenotropic murine leukemia virus (X-MuLV), bovine viral diarrhea virus (BVDV), Pseudorabies virus (PRV), Feline Calicivirus (FCV), and Porcine Parvovirus (PPV).
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The active ingredient in SEVENFACT is a recombinant analog of human Factor VIIa, a vitamin K-dependent coagulation factor. In the presence of both calcium and phospholipids, Factor VIIa in a complex with tissue factor (TF) activates Factor X to Factor Xa, directly bypassing the reactions that require Factor VIII or Factor IX. Activation of Factor X to Factor Xa initiates the common pathway of the coagulation cascade in which prothrombin is activated to thrombin, which then converts fibrinogen to fibrin to form a hemostatic plug, thereby achieving clot formation at the site of hemorrhage (hemostasis). This process may also occur in the absence of TF on the surface of activated platelets.
12.2 Pharmacodynamics
Laboratory assessment of coagulation does not necessarily correlate with or predict the hemostatic effectiveness of SEVENFACT.
SEVENFACT demonstrated a dose and concentration-dependent pharmacodynamics effect on the coagulation system, including shortening of the activated partial thromboplastin time (aPTT) and the prothrombin time (PT), and increasing the thrombin generation with platelets (TGT) and the maximum clot firmness (ROTEM-FIBTEM test).
12.3 Pharmacokinetics
Pharmacokinetic (PK) evaluation was conducted in a Phase 3, global, multicenter, prospective, open-label, randomized, crossover study (Study 1). PK analysis was conducted in subjects after a single intravenous administration of either 75 mcg/kg or 225 mcg/kg of SEVENFACT as a bolus injection.
| PK Parameter
(Arithmetic Mean [CV%]) | Cmax
(ng/mL) | Clearance
(L/h) | Vss
(L) | AUC0-inf
(ng*h/mL) | t1/2
(h) |
| 75 mcg/kg (n=6) | 566.2 (71.4) | 8.0 (44.6) | 19.9 (47.0) | 589.1 (44.4) | 1.7 (13.2) |
| 225 mcg/kg (n=5) | 2440.6 (22.2) | 5.8 (17.1) | 11.9 (21.5) | 2841.2 (20.0) | 1.4 (12.2) |
Five minutes after infusion, plasma SEVENFACT levels were 566.2 ng/mL and 2440.6 ng/mL for 75 mcg/kg and 225 mcg/kg dose groups respectively. Observed plasma concentration-time profiles show a biexponential decay from the maximal concentration to return to baseline approximately 8 hours post-administration. Exposure PK parameters Cmax and AUC0-inf suggested dose dependence over the ranges studied. The clearance of SEVENFACT were 8.0 L/h and 5.8 L/h for 75 mcg/kg and 225 mcg/kg dose levels respectively. Half-life time is approximately 1.6 hours (1.4 hours for 225 mcg/kg and 1.7 hours for 75 mcg/kg utilizing Non-Compartmental Analysis).
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals to evaluate the potential effect of SEVENFACT on carcinogenesis or mutagenesis were not conducted. Male rats intravenously infused with SEVENFACT at 0.1, 0.3, 1 and 3 mg/kg/day (up to 13-fold higher than the highest recommended human dose of 225 mcg/kg), beginning 28 days before cohabitation and during cohabitation did not display adverse effects for the mating index, fertility, or conception rate. Comparing animals administered vehicle to animals administered SEVENFACT, there were no differences in sperm motility, morphology, or concentration.
13.2 Animal Toxicology and/or Pharmacology
The no observed adverse effect level (NOAEL) in 28-day repeat-dose toxicity studies in male Sprague-Dawley rats and Cynomolgus monkeys was 1 mg/kg/day, which is 4-fold higher than the highest recommended human dose. Anti-drug antibody formation was observed by Day 29 in both animal species. The NOAEL in a 13-week repeat-dose toxicity study in male and female Cynomolgus monkeys was 1 mg/kg/day. Anti-drug antibody formation was observed in all animals by Day 28. Antibody presence was not associated with any adverse effects in either animal species.
14 CLINICAL STUDIES
The efficacy and safety of SEVENFACT for treatment of bleeding episodes was evaluated in Study 1. Twenty-seven subjects with hemophilia A or B with inhibitors were treated for 468 bleeding events, of which 465 were mild or moderate and three were severe bleeding events. Of the 27 subjects, 5 subjects were 12 to < 18 years of age and they experienced 79 bleeding events. The study was a global, multicenter, randomized, open-label, crossover of two initial dose regimens. Subjects were male, predominantly Caucasian (93%), with a mean age of 31 (range 12-54) years and median of 10 (3-50) bleeding episodes in six months prior to study enrollment. Overall, target joint(s)/bleeding site(s) were reported in 63% of subjects at study entry.
All doses of SEVENFACT were given at home or in clinic.
Of the 468 bleeding events in diverse anatomic locations that were treated, 82% were spontaneous and the remaining (18%) were traumatic bleeding episodes; 465 were mild or moderate bleeding events and 3 were severe (refer to Table 5). The majority (98%) of bleeding events were treated at home, with 88% treated within one hour of recognition of bleeding.
Treatment Regimens for Mild or Moderate Bleeding Episodes
For mild to moderate bleeding episodes, subjects were randomized to one of two initial dose regimens of SEVENFACT:
- 75 mcg/kg followed by subsequent doses of 75 mcg/kg every 3 hours as necessary to achieve hemostatic efficacy. A total of 8 administrations were allowed in this dose regimen.
- 225 mcg/kg dose followed 9 hours later with 75 mcg/kg doses every 3 hours as necessary to achieve hemostatic efficacy. A total of 6 administrations were allowed in this dose regimen.
Treatment with SEVENFACT was discontinued when bleeding persisted 24 hours after the first administration of SEVENFACT.
In Study 1, subjects were randomized to one of two initial dosing regimens and continued on the dose regimen for three months, after which the subjects were crossed over to the other dose regimen for three months.
Treatment Regimens for Severe Bleeding Episodes
Patients in the randomized study who experienced a severe bleed were administered the initial dose of 225 mcg/kg SEVENFACT at home, and were required to receive subsequent treatments at 75 mcg/kg every two hours in a hospital or hemophilia treatment center (HTC), if additional doses were considered necessary for treatment of ongoing bleeding. If response to treatment after the first or any subsequent administrations of study drug were satisfactory (i.e., efficacy assessment was rated as “good” or “excellent”), the dosing interval was changed from two hours to three hours for 1 to 2 days, after which the interval could be increased to 4 to 12 hours, depending on the severity of the bleeding episode, for as long as needed.
Bleeding Assessment
The primary endpoint of the study was successful treatment of mild or moderate bleeding episode at 12 hours after initial SEVENFACT dose administration. Success was defined by a combination of the following: subject’s response of “good” or “excellent” using a 4-point hemostatic efficacy scale (presented in Table 4), no further treatment with SEVENFACT beyond the 12-hour time point, no other hemostatic treatment needed for the bleeding episode, no administration of blood products, and no increase in pain beyond 12 hours.
| Patient and/or *HCP Evaluation | Therapeutic Response | Description |
| None | Lack of hemostatic efficacy | No noticeable effect of the treatment on the bleeding or worsening of subject’s condition. Continuation of treatment with the study drug was needed. |
| Moderate | Lack of hemostatic efficacy | Some effect of the treatment on the bleeding was noticed (e.g., pain decreased, or bleeding signs improved) but the bleeding continued and required continued treatment with the study drug. |
| Good | Hemostatic efficacy | Symptoms of the bleeding (e.g., swelling, tenderness, and decreased range of motion in the case of musculoskeletal hemorrhage) had largely been reduced by the treatment, but had not completely disappeared. Symptoms had improved enough to not require more infusions of the study drug. |
| Excellent | Hemostatic Efficacy | Full relief of pain and cessation of objective signs of the bleeding (e.g., swelling, tenderness, and decreased range of motion in the case of musculoskeletal hemorrhage). No additional infusion of study drug was required. |
| *Healthcare provider | ||
The primary efficacy analysis compared hemostatic efficacy of each dosing regimen with a prespecified objective performance criterion (OPC) of 55%. This OPC was based on historical data for hemostatic efficacy of bypassing agents. The study was powered to detect a 15% improvement over OPC for each dosing regimen. Results of the primary efficacy analysis are shown in Table 5.
Of the 465 mild or moderate bleeding episodes, 17 bleeding events were not evaluable due to missing a hemostatic efficacy assessment at 12 hours.
The proportion of mild or moderate bleeding events with hemostatic efficacy at 12 hours was 82% in the 75 mcg/kg dose regimen group and was 91% in the 225 mcg/kg dose regimen group.
Hemostatic efficacy was evaluated in 79 bleeding events in the five adolescent subjects: for the 75 mcg/kg dose regimen, hemostatic efficacy was 93% (95% CI; 81%- 99%) and for the 225 mcg/kg dose regimen, it was 91% (95% CI; 77%-98%), with the confidence intervals given by the Clopper-Pearson exact method.
| 75 mcg/kg
(N=25)1 | 225 mcg/kg
(N=25)1 | Overall
(N=27)1 |
|
| Number of bleeding episodes | 252 | 213 | 465 |
| Number of bleeds with hemostatic efficacy | 197 (78.1%) | 188 (88.2%) | 385 (82.8%) |
| Number of failures | 44 (17.4.%) | 19 (8.9%) | 63 (13.5%) |
| Number of missing | 11 (4.3%) | 6 (2.8%) | 17 (3.7%) |
| Proportion of bleeds with hemostatic efficacy [95% CI]2 | 82%
[72%, 91%] | 91%
[84%, 98%] | 86%
[78%, 93%] |
| p-value3 | <0.001 | <0.001 | |
| 1 N in the column header indicates number of subjects who had at least 1 bleeding episode treated with a given dose of study drug. 2 Analysis was based on data as observed. No missing value imputation was made. 3 p-value from 1-sided normal approximation test of H0: p ≤0.55, where p is the true proportion of successfully treated mild or moderate bleeding episodes at 12 hours, with adjustment for the correlation among bleeding episodes for a given subject. The null hypothesis of hemostatic efficacy less than or equal to 55% was rejected. Note: Stratified by actual treatment regimen at the time of the bleeding episode. CI = confidence interval. |
|||
The median and mean (SD) numbers of administrations per mild or moderate bleeding episode were 2.0 and 2.5 (1.75) for the 75 mcg/kg dose regimen and 1.0 and 1.4 (0.96) for the 225 mcg/kg dose regimen.
The median time to attain good or excellent assessment by the patient was 6 hours for the 75 mcg/kg dose regimen and 3 hours for the 225 mcg/kg dose regimen.
There were 3 severe bleeding episodes, of which one was a traumatic intramuscular bleeding episode and two were spontaneous bleeding episodes in the right hip and kidney. Hemostasis was achieved at 12 hours in the three severe bleeding events. One severe bleed was treated with three 225 mcg/kg doses administered every 6 hours, which was a deviation from the study protocol-specified dosing. The remaining 2 subjects were treated with 1 and 5 doses of SEVENFACT respectively.
No subject received any alternative therapy prior to 24 hours. In addition, 97.6% of bleeding episodes treated with the 75 mcg/kg dose regimen, and 99.5% of bleeding episodes treated with the 225 mcg/kg dose regimen, did not require treatment with alternative bypassing agents.
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
- SEVENFACT [coagulation factor VIIa (recombinant)-jncw], is supplied as a room temperature stable, white to off-white, lyophilized powder in single-use vials, one vial per carton. The diluent for reconstitution of SEVENFACT is Water for Injection supplied as a clear colorless solution in a pre-filled syringe.
- Single 1 or 5 mg vials of SEVENFACT are available in packages as indicated below.
| Presentation | Cap Color Indication | NDC Number | Contents |
| 1 mg per vial | Yellow | NDC 71127-1000-1 |
|
| 5 mg per vial | Purple | NDC 71127-5000-1 |
|
- The SEVENFACT vials are made of glass, closed with a bromobutyl rubber stopper (not made with natural rubber latex), and sealed with an aluminum cap.
- The pre-filled diluent syringes are made of glass, with a siliconized bromobutyl rubber plunger (not made with natural rubber latex).
Storage and Handling
- Prior to reconstitution, the SEVENFACT kit should be stored at room temperature but can be stored between 36°F to 86°F (2°C to 30°C), protected from light in the product package. Do not freeze.
- After reconstitution, SEVENFACT should be stored at room temperature but can be stored between 36°F to 86°F (2°C to 30°C), for up to 4 hours. Do not freeze or store in syringes.
17 PATIENT COUNSELING INFORMATION
Advise patients:
- to read the FDA-approved patient labeling (Patient Product Information and Instructions for Use).
- about the early signs of hypersensitivity reactions and to seek medical help if the following occur:
- Hives, itching, rash, difficulty breathing, swelling around the mouth and throat, tightness of the chest, wheezing, dizziness or fainting, low blood pressure, or other symptoms of anaphylaxis.
- about the signs of thrombosis and to seek medical help if the following occur:
- New-onset swelling and pain in the limbs or abdomen, new-onset chest pain, shortness of breath, loss of sensation or motor power, or altered consciousness or speech.
Revised: 11/2022
License Number: 2061
“SEVENFACT” is a trademark of LFB S.A.
PATENT Information: https://hemabiodup.wpengine.com/patents/
For information contact:
Call: 855.718.HEMA (4362)
Fax: 855.721.HEMA (4362)
Email: medinfo@hemabio.com
Manufactured by:
Laboratoire Français du Fractionnement et des Biotechnologies S.A. (LFB S.A.)
Puteaux, 92800
France
U.S. License Number: 2061
Distributed by:
HEMA Biologics
Louisville, KY 40241
Patient Product Information
SEVENFACT® (SEV-en-fact)
coagulation factor VIIa (recombinant)-jncw
For Intravenous Injection After Reconstitution Only
PLEASE READ PATIENT PRODUCT INFORMATION AND THE INSTRUCTIONS FOR USE THAT COME WITH SEVENFACT BEFORE YOU START TAKING THIS MEDICINE AND EACH TIME YOU GET A REFILL. THERE MAY BE NEW INFORMATION.
This Patient Product Information does not take the place of talking with your healthcare provider about your medical condition and treatment. If you have questions about SEVENFACT after reading this information, ask your healthcare provider.
What is the most important information I should know about SEVENFACT?
The most serious possible side effect of SEVENFACT is abnormal blood clotting involving blockage of blood vessels, which include stroke, blockage of the main blood vessel to the lung, and deep vein blood clots.
You should know the signs of abnormal clotting (thrombosis) described below and seek medical help immediately if they occur.
New onset of swelling and pain in the limbs or abdomen, new onset of chest pain, shortness of breath, loss of sensation or motor power, and altered consciousness or speech can all be signs of clot formation in places other than your site of bleeding. Seek immediate medical attention if you experience one or more of these symptoms.
SEVENFACT should be used as prescribed and directed by your healthcare provider.
What is SEVENFACT?
SEVENFACT is a recombinant human Factor VIIa protein for injection. SEVENFACT can allow adolescents and adults with Hemophilia A or B with inhibitors to create clotting at the site of bleeding without needing Coagulation Factor VIII or IX replacement.
SEVENFACT, coagulation factor VIIa (recombinant)-jncw, is indicated for the treatment and control of bleeding episodes occurring in adults and adolescents 12 years of age and older with Hemophilia A or B with inhibitors.
Injecting medications requires special training. Do not attempt to self-infuse unless you have been taught how by your health care provider or hemophilia treatment center. Once trained, you will need additional infusion materials along with your SEVENFACT so that you can successfully treat your bleeding episodes at home. Be sure to collect all necessary infusion materials before starting the reconstitution process.
SEVENFACT comes in a sterile dry powdered dosage form that must be reconstituted with sterile Water for Injection.
It is not known if SEVENFACT is safe and effective in children under 12 years of age.
Who should not USE SEVENFACT?
You should not use SEVENFACT if you:
- Are allergic to rabbits.
- Have known allergies to SEVENFACT or any of its components.
Tell your doctor prior to infusing SEVENFACT if you have begun treatment of a bleeding episode with another bypassing agent such as activated prothrombin complex concentrate (FEIBA®).
What should I tell my healthcare provider before I use SEVENFACT?
Tell your healthcare provider if you:
- Are pregnant, planning to become pregnant or nursing, as SEVENFACT has not been studied in patients with Hemophilia A or B with inhibitors who are pregnant or nursing.
- Had prior blood clots, heart disease, heart failure, abnormal heart rhythms, prior pulmonary clots or heart surgery.
- Have or have had any other medical conditions.
You and your doctor can then decide whether SEVENFACT is the right treatment for you, as well as the proper timing and doses you will need for SEVENFACT to control your bleeding episodes at home.
How should I use SEVENFACT?
Treatment with SEVENFACT should be started by a healthcare provider who is experienced in the care of patients with Hemophilia A or B with inhibitors.
SEVENFACT is given as an injection into your vein.
You may infuse SEVENFACT at a hemophilia treatment center, at your healthcare provider’s office, or in your home. You should be trained on how to infuse by your healthcare provider or hemophilia treatment center. Many people with inhibitors learn to infuse by themselves or with the help of a family member.
Treating at first sign of a bleed is important for bleed management. Your healthcare provider will tell you how much SEVENFACT to use based on your weight and when to administer SEVENFACT.
To administer SEVENFACT:
- Collect all materials needed for your prescribed dose
- Follow the Instructions For Use guide to reconstitute the prescribed number of SEVENFACT vials
- Infuse following your healthcare provider’s instructions, using infusion materials from your pharmacy
Contact your healthcare provider if you:
- Miss a dose, or
- Administer more than your prescribed dose, or
- Think your bleed is not controlled within the expected time frame discussed with your healthcare provider.
What should I avoid while using SEVENFACT?
- Avoid activity that can create more bleeding once you have completed your SEVENFACT infusion
- Avoid mixing SEVENFACT with other medications
- Avoid infusing SEVENFACT and other factor-containing therapies [such as activated prothrombin complex concentrate (aPCC) or other recombinant Factor VIIa products] at the same time. This increases your risk of having a disabling blood clot.
What are the possible side effects of SEVENFACT?
The most common adverse reactions reported in clinical trials for SEVENFACT were headache, dizziness, infusion-site discomfort, infusion-site hematoma, infusion-related reaction and fever.
A serious allergic reaction to SEVENFACT may occur. If you experience the severe symptoms of an allergic reaction after infusing SEVENFACT, seek immediate medical attention. Severe symptoms occur when your immune system reacts very strongly to foreign proteins or drugs.
- Hives, itching, rash, difficulty breathing with cough or wheezing, swelling around the mouth and throat, tightness of the chest, dizziness or fainting, and low blood pressure are all symptoms of a severe allergic reaction (anaphylaxis). Call 911 should you experience one or more of these symptoms.
These are not all the possible side effects of SEVENFACT. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store SEVENFACT?
- SEVENFACT should be stored in its product packaging to protect from light.
- Prior to reconstitution, the SEVENFACT kit should be stored at room temperature but can be stored between 36°F to 86°F (2°C to 30°C).
- After reconstitution, SEVENFACT should be stored at room temperature but can be stored between 36°F to 86°F (2°C to 30°C), for up to 4 hours.
- SEVENFACT should not be frozen.
General information about the safe and effective use of SEVENFACT
SEVENFACT is sometimes prescribed for purposes other than those listed here.
This leaflet summarizes the most important information about SEVENFACT.
Do not use SEVENFACT for a condition for which it was not prescribed. Do not give SEVENFACT to other people even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about SEVENFACT that is written for health professionals.
For more information, go to www.SEVENFACT.com or call 855.718.HEMA (4362).
What are the ingredients in SEVENFACT?
Active ingredient: coagulation factor VIIa (recombinant)-jncw
Inactive ingredients: arginine hydrocholoride, glycine, isoleucine, lysine hydrochloride, polysorbate 80, trisodium citrate dihydrate, hydrochloric acid, nitrogen and water for injection.
For Information Contact:
HEMA Biologics, LLC
4441 Springdale Road
Louisville, Kentucky 40241-1086
Manufactured by:
Laboratoire Francais du Fractionnement et des Biotechnologies S.A. (LFB S.A.)
Puteaux, 92800
France
Distributed by:
HEMA Biologics
Louisville, KY 40241
U.S. License No. 2061
All trademarks are the property of their respective owners.
“SEVENFACT” is a trademark of LFB S.A.
PATENT Information: https://hemabiodup.wpengine.com/patents/
Revised: 11/2022


INSTRUCTIONS FOR USE:
READ BEFORE YOU START USING SEVENFACT®
Your healthcare provider should show you and/or your caregiver how to reconstitute and administer SEVENFACT® the first time it is used. Use aseptic techniques.
Your SEVENFACT® kit contains:

Vial Adapters* and Packaging
1 mg vial adapter 5 mg vial adapter

*Note: Each SEVENFACT kit will contain only one vial adapter.
You will also need:
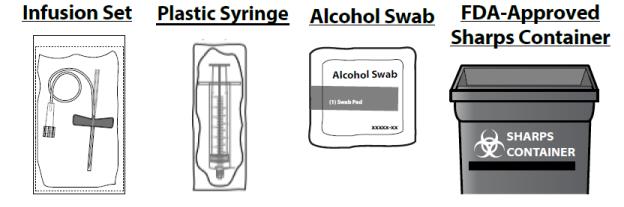
- Collect Supplies and Prepare Vial
- Take out the number of SEVENFACT® kits you need to fulfill your prescribed dose, an infusion set (not supplied) and an alcohol swab (not supplied).
Do not use the kit if the tamper seal has been broken or you suspect the kit is contaminated. Use a new kit instead.
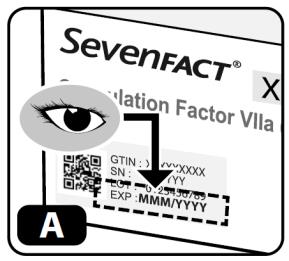
- Check the expiration date on the side of the kit (Figure A).
Do not use if expired.
- Clean a flat surface before starting the steps for reconstituting SEVENFACT®.
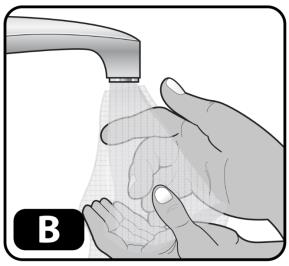
- Wash your hands with soap and water and dry using a clean towel or air dry (Figure B).
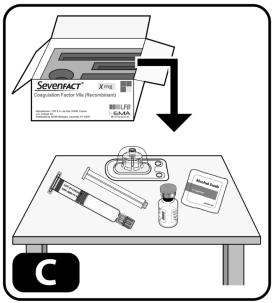
- Take out the contents of one kit and one alcohol swab. Place items on the clean surface (Figure C).
- Inspect all contents of the kit. Make sure each vial has a matching colored syringe.
Do not use the contents if they have been dropped or if they are damaged. Use a new kit instead.
- If not already at room temperature, bring the vial and the pre-filled syringe to room temperature. You can do this by holding them in your hands until they feel as warm as your hands.
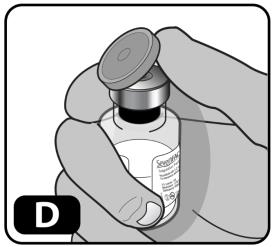
- Remove the plastic cap from the vial (Figure D).
If the plastic cap is loose or missing, do not use the vial.
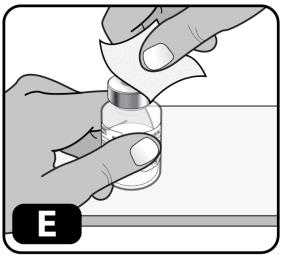
- Wipe the rubber stopper with an alcohol swab (Figure E) and allow it to air dry for a few seconds to ensure that it is as germ free as possible.
- After cleaning with the swab, do not touch the rubber stopper with your fingers and do not allow it to touch any other object until you attach the vial adapter, as this can transfer germs.
2 Attach Vial Adapter
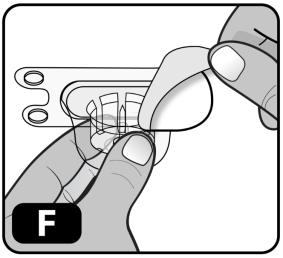
- Peel off the paper protective cover from the vial adapter package (Figure F).
- Hold the vial on the clean flat surface with one hand, using your other hand to hold the plastic cover (with the vial adapter inside) directly over the drug vial. The spike of the adapter should line up with the middle of the gray rubber stopper.
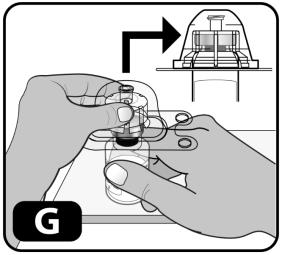
- Firmly press down so the vial adapter spike breaks through the rubber stopper (you may hear/see it "snap" into place) (Figure G).
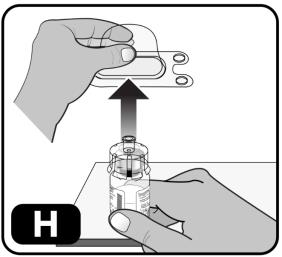
- Lightly squeeze the plastic cover and lift up to remove it from the vial adapter (Figure H).
Do not touch the top of the vial adapter once the plastic cover is removed to avoid transferring germs from your fingers.
NOTE: The 5 mg vial adapter may not sit flat against the vial, but it is fully functional.
3 Attach Pre-filled Syringe and Install the Plunger Rod
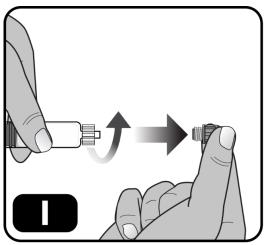
- Remove the syringe cap from the pre-filled syringe by holding the syringe body with one hand and using the other hand to unscrew the syringe cap (turn to the left) (Figure I).
Do not touch the syringe tip under the syringe cap to avoid transferring germs from your fingers.
Do not use the pre-filled syringe if the syringe cap is loose or missing.
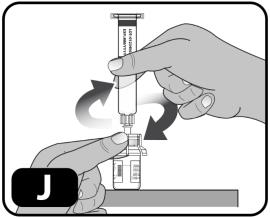
- While holding the edges of the vial adapter, screw on the pre-filled syringe (turn to the right) a few turns until it starts to tighten (Figure J).
Be careful not to overtighten as you will need to remove the syringe later.
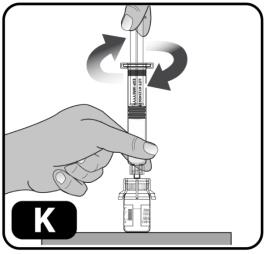
- Hold the plunger rod by the wide top end in one hand and the syringe body using your other hand.
- Insert the plunger rod into the syringe, then screw a few turns (turn to the right) so that the plunger rod is attached to the gray rubber stopper in the syringe (Figure K).
4 Mix Drug in Vial
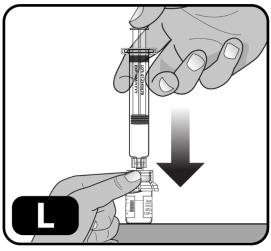
- Very slowly push down on the plunger rod to the bottom of the syringe, to transfer all of the liquid from the syringe into the drug vial (Figure L).
Do not push too quickly as it can result in excess foam and air in the vial.
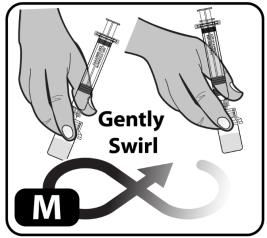
- Swirl the vial gently or roll between hands until all powder is dissolved (Figure M).
Do not shake the vial as this creates foam and air.
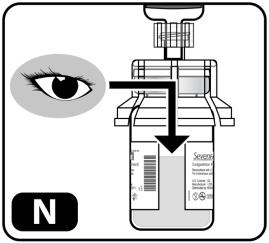
- Check the mixed solution (Figure N). It should be clear to slightly opaque. All powder should be dissolved with no particles floating in the liquid.
Do not use the drug if liquid has particles or is cloudy after mixing. Start over with a new kit.
5 Remove Empty Syringe from Vial Adapter
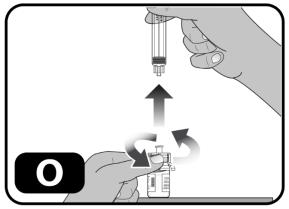
- Without withdrawing any drug back into the syringe, unscrew the syringe from the vial adapter (turn to the left) until it is completely detached (Figure O).
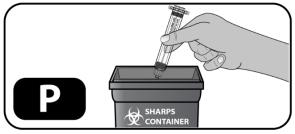
- Throw out empty syringe into an FDA-approved sharps container (Figure P).
Do not remove the vial adapter.
Do not touch the luer lock top of the vial adapter. If you touch this top, germs from your fingers can be transferred.
6 Mix Additional Vial(s) and Infuse Dose
- If your dose requires more than one vial, repeat the above steps with additional kits until you have reached your required dose.
- Withdraw the liquid drug from the vial(s), using a syringe provided by your pharmacy that is large enough to hold your prescribed dose.
- SEVENFACT® must be infused within 4 hours of reconstitution (Figure Q).
Do not use if more than 4 hours have passed since reconstitution.
- SEVENFACT® can be infused in 2 minutes or less as an intravenous infusion per the instructions of your healthcare provider.
7 Throw Out Empty Drug Vial(s)
- After reconstitution and infusion, safely dispose of the vial with vial adapter attached, the infusion syringe, and any other waste materials into an FDA-approved sharps container (Figure R).
Do not throw out with the ordinary household trash.
Do not disassemble the vial and vial adapter before disposal.
Do not reuse any components of the kit.
- If you do not have an FDA-approved sharps container, you may use a container with the following:
-
Made of heavy duty plastic,
-
Can be closed, puncture resistant and prevents sharps from coming out,
-
Upright and stable,
-
Leak resistant,
- Properly labeled as hazardous
-
Made of heavy duty plastic,
Follow local or state guidelines for proper disposal of sharps container. For more information about safe sharps disposal visit FDA’s website: http://www.fda.gov/safesharpsdisposal
Storage
SEVENFACT® is supplied in a kit that should be stored at room temperature, but can be stored between 36°F to 86°F (2°C-30°C).
Do not open the kit contents until you are ready to use them.
Do not freeze or store in syringes reconstituted SEVENFACT® solution.
Avoid exposure of the reconstituted SEVENFACT® solution to direct light.
Important Information
SEVENFACT® is for intravenous infusion only. Do not administer through any other route (subcutaneous or intramuscular).
Contact your healthcare provider or local hemophilia treatment center if you experience problems.
For questions or complaints:
Call: 855.718.HEMA (4362)
Fax: 855.721.HEMA (4362)
or please contact your HEMA Biologics representative for assistance.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by LFB S.A. Puteaux, 92800, France
Distributed by HEMA Biologics, Louisville, KY 40241
Revised: 11/2022
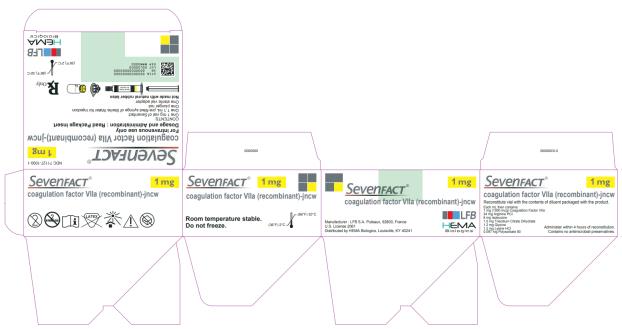
PRINCIPAL DISPLAY PANEL
NDC 71127-1000-1
SEVENFACT® 1 mg
coagulation factor VIIa (recombinant)-jncw
For intravenous use only
Dosage and Administration: Read Package Insert
CONTENTS:
One 1 mg vial of Sevenfact
One 1.1 mL pre-filled syringe of Sterile Water for Injection
One plunger rod
One sterile vial adapter
Not made with natural rubber latex
Rx only
LFB
HEMA Biologics
PRINCIPAL DISPLAY PANEL
NDC 71127-5000-1
SEVENFACT® 5 mg
coagulation factor VIIa (recombinant)-jncw
For intravenous use only
Dosage and Administration: Read Package Insert
CONTENTS:
One 5 mg vial of Sevenfact
One 5.2 mL pre-filled syringe of Sterile Water for Injection
One plunger rod
One sterile vial adapter
Not made with natural rubber latex
Rx only
LFB
HEMA Biologics

PRINCIPAL DISPLAY PANEL
NDC 71127-1100-1
SEVENFACT® 1 mg
coagulation factor VIIa (recombinant)-jncw
Reconstitute with 1.1 mL Sterile Water for Injection
For intravenous administration only
Store between 2-30°C (36-86°F). Rx only
Manufactured by LFB S.A. U.S. License No. 2061

PRINCIPAL DISPLAY PANEL
NDC 71127-5100-1
SEVENFACT® 5 mg
coagulation factor VIIa (recombinant)-jncw
Reconstitute with 5.2 mL Sterile Water for Injection
For intravenous administration only
Store between 2-30°C (36-86°F). Rx only
Manufactured by LFB S.A. U.S. License No. 2061