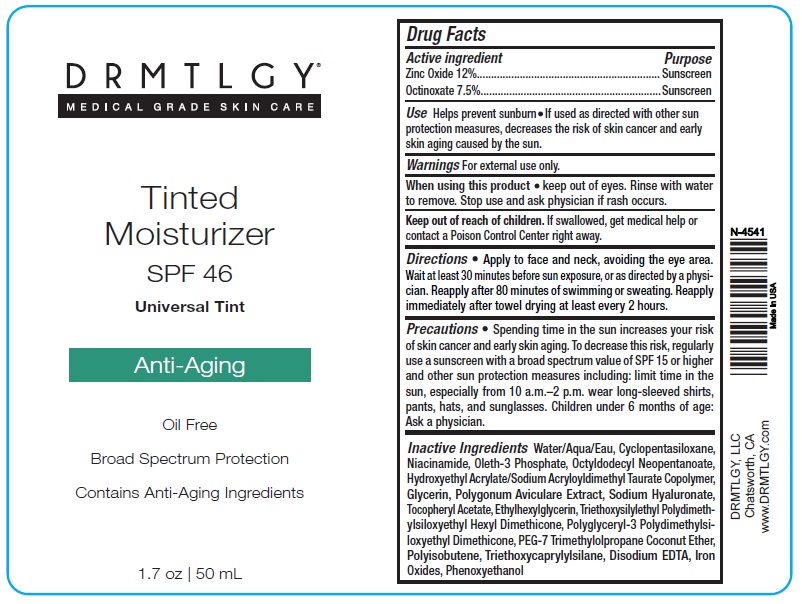
Use Helps prevent sunburn • If used as directed with other sun protection measures, decreases the risk of skin cancer and early skin aging caused by the sun.
Warnings For external use only.
When using this product • keep out of eyes. Rinse with water to remove. Stop use and ask physician if rash occurs.
Directions • Apply to face and neck, avoiding the eye area. Wait at least 30 minutes before sun exposure, or as directed by a physician. Reapply after 80 minutes of swimming or sweating. Reapply immediately after towel drying at least every 2 hours.
Precautions • Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum value of SPF 15 or higher and other sun protection measures including: limit time in the sun, especially from 10 a.m.–2 p.m. wear long-sleeved shirts, pants, hats, and sunglasses. Children under 6 months of age: Ask a physician.
Inactive Ingredients Water/Aqua/Eau, Cyclopentasiloxane, Niacinamide, Oleth-3 Phosphate, Octyldodecyl Neopentanoate, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Glycerin, Polygonum Aviculare Extract, Sodium Hyaluronate, Tocopheryl Acetate, Ethylhexylglycerin, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, PEG-7 Trimethylolpropane Coconut Ether, Polyisobutene, Triethoxycaprylylsilane, Disodium EDTA, Iron Oxides, Phenoxyethanol