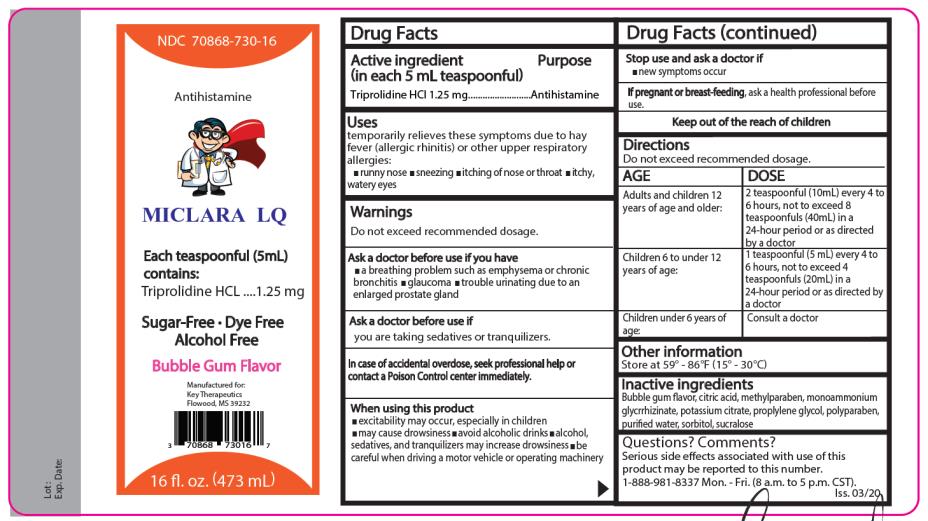
Miclara LQ- triprolidine hydrochloride liquid
Key Therapeutics
Disclaimer: Most OTC drugs are not reviewed and approved by FDA; however, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Uses
temporarily relieves these symptoms due to hay fever (allergic rhinitis) or other upper respiratory allergies:
- runny nose
- sneezing
- itching of the nose or throat itchy,
- watery eyes
Warnings
Do not exceed recommended dosage.
Do not use this product if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
When using this product
- may cause excitability especially in children
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase the drowsiness effect
- be careful when driving a motor vehicle or operating machinery
Directions
Do not exceed recommended dosage.
Adults and children
12 years of age and older:
Children 6 to under 12 years of age:
2 teaspoonfuls (10 mL) every 4 to 6 hours, not to exceed
8 teaspoonfuls (40mL) in 24-hour
period or as directed by a doctor.
1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 4 teaspoonfuls (20mL) in a 24-hour period or as directed by a doctor.
Children under 6 years of age: Consult a doctor
Inactive ingredients
Bubble gum flavor, citric acid, methylparaben, monoammonium glycyrrhizinate, potassium citrate, propylene glycol, propylparaben, purified water, sorbitol, sucralose.