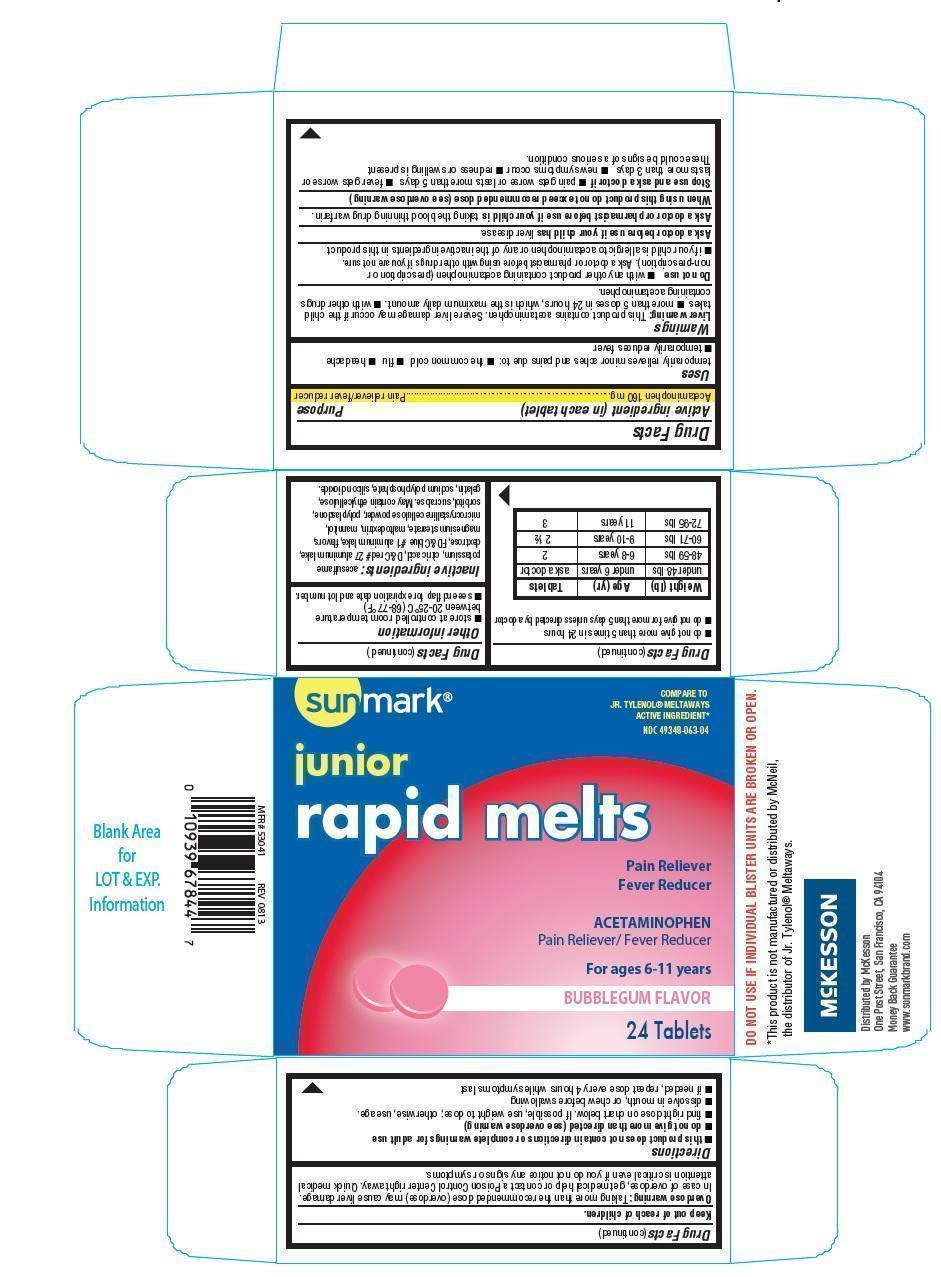
Uses
temporarily relieves minor aches and pains due to:
- the common cold
- flu
- headache
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if the child takes
- more than 5 doses in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen.
Do not use
- with any other product containing acetaminophen (prescription or nonprescription). Ask a doctor or pharmacist before using with other drugs if you are not sure.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product.
Ask a doctor before use if your child has
liver disease
Ask a doctor or pharmacist before use if your child is
taking the blood thinning drug warfarin
When using this product do not exceed recommended dose (see overdose warning)
Stop use and ask a doctor if
- pain gets worse or lasts more than 5 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition
Keep out of reach of children.
Overdose warning: Thaking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a poison control center right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
Directions
- this product does not contain directions or complete warnings for adult dose
- do not give more than directed (see overdose warning)
- find right dose on chart below. If possible, use weight to dose; otherwise, use age.
- dissolve in mouth, or chew before swallowing
- if needed, repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give more than 5 days unless directed by a doctor.
| Weight (lb) | Age (yr) | Tablets |
| under 48 lbs | under 6 years | ask a doctor |
| 48-59 lbs | 6-8 years | 2 |
| 60-71 lbs | 9-10 years | 2 1/2 |
| 72-95 lbs | 11 years | 3 |
Other information
- store at controlled room temperature betweeen 20-25oC(68-77oF)
- see end flap for expiration date and lot number
Inactive ingredients
acesulfame pottasium, citric acid, D&C red 27 aluminum lake, dextrose, FD&C blue 1 aluminum lake, flavors, magnesium stearate, maltodextrin, mannitol, microcrystalline cellulose powder, polyplasdone, sorbitol, sucralose.
May contain ethylcellulose, gelatin, sodium polyphosphate, silicon dioxide.