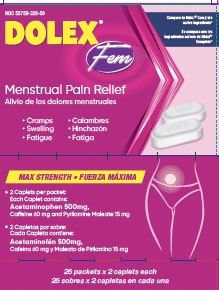
Uses
For the temporary relief of these symptoms associated with menstrual periods
- cramps
- bloating
- water- weight gain
- headache
- backache
- Muscle aches
- fatigue
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 6 caplets in 24 hours, which is the maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product.
Allergy alert: Acetaminophen may cause severe skin or severe allergic reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin or general allergic reaction occurs, stop use and seek medical help right away.
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability,sleeplessness, and, occasionally, rapid heart beat.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- for more than 10 days for pain unless directed by a doctor
- if allergic to any of the ingredients in this product
Ask a doctor before use if you have
- liver disease
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
Directions
- do not exceed recommended dose
- take every
6 hours. Do not exceed 6 caplets in a 24-hour period
- adults and children 12 years of age and over: take two (2) tablets, every 6 hours
- children under 12 years of age: do not use
Inactive ingredients
corn starch, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone k30, silicon dioxide, sodium starch glycolate, stearic acid, talc, titanium dioxide