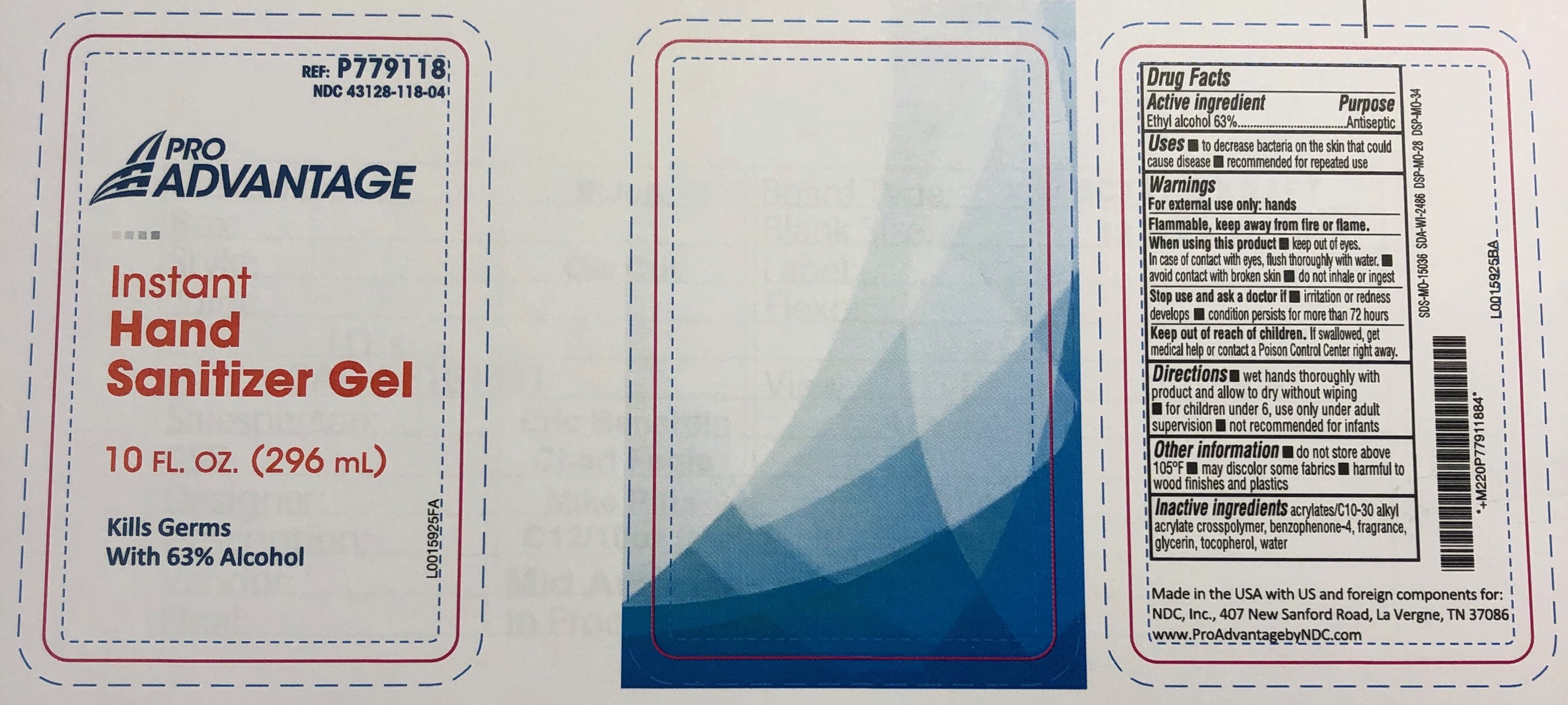
PRO ADVANTAGE INSTANT HAND SANITIZER- alcohol gel
NDC National Distribution & Contracting, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Pro Advantage Instant Hand Sanitizer Gel
Active Ingredients:
Alcohol 62%
Use
To help reduce bacteria on the hands that can potentially cause disease.
Warning:
• For External Use Only
• Flammable, keep away from fire or flame.
When using this product
• Avoid contact with eyes, if this occurs rinse thoroughly with water and contact a physician.
Ask a doctor before use if you have
• Deep wounds, animals bites or serious burns.
Stop use and ask a doctor if
• Condition persists.
Keep out of reach of children.
• If swallowed get medical help or contact a Poison Control Center right away.
Directions:
Apply a liberal amount to hands and rub hands thoroughly until dry. Do not rinse or wipe off gel.
Inactive Ingredients:
Water, Glycerin, Fragrance, Carbomer, Triethanolamine, DMDM Hydantoin
REF: P779118
NDC 43128-118-04
Made in China
www.ProAdvantagebyNDC.com
Manufactured for NDC, Inc.
407 New Sanford Road, La Vergne, TN 37086
Product Labels

NDC National Distribution & Contracting, Inc.