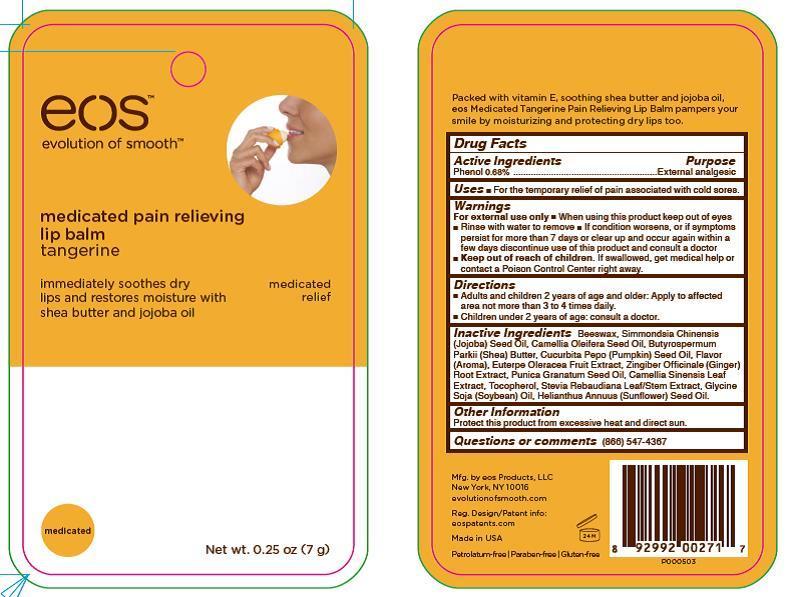
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
If condition worsens, or if symptoms persist for more than 7 days or clear up and occur again within a few days discontinue use of this product and consult a doctor.
Directions
Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
Children under 2 years of age: consult a doctor.
Inactive Ingredients
Beeswax, Simmondsia Chinensis (Jojoba) Seed Oil, Camellia Oleifera Seed Oil, Butyrospermum Parkii (Shea) Butter, Cucurbita Pepo (Pumpkin) Seed Oil, Flavor (Aroma), Euterpe Oleracea Fruit Extract, Zingiber Officinale (Ginger) Root Extract, Punica Granatum Seed Oil, Camellia Sinensis Leaf Extract, Tocopherol, Stevia Rebaudiana Leaf/Stem Extract, Glycine Soja (Soybean) Oil, Helianthus Annuus (Sunflower) Seed Oil.