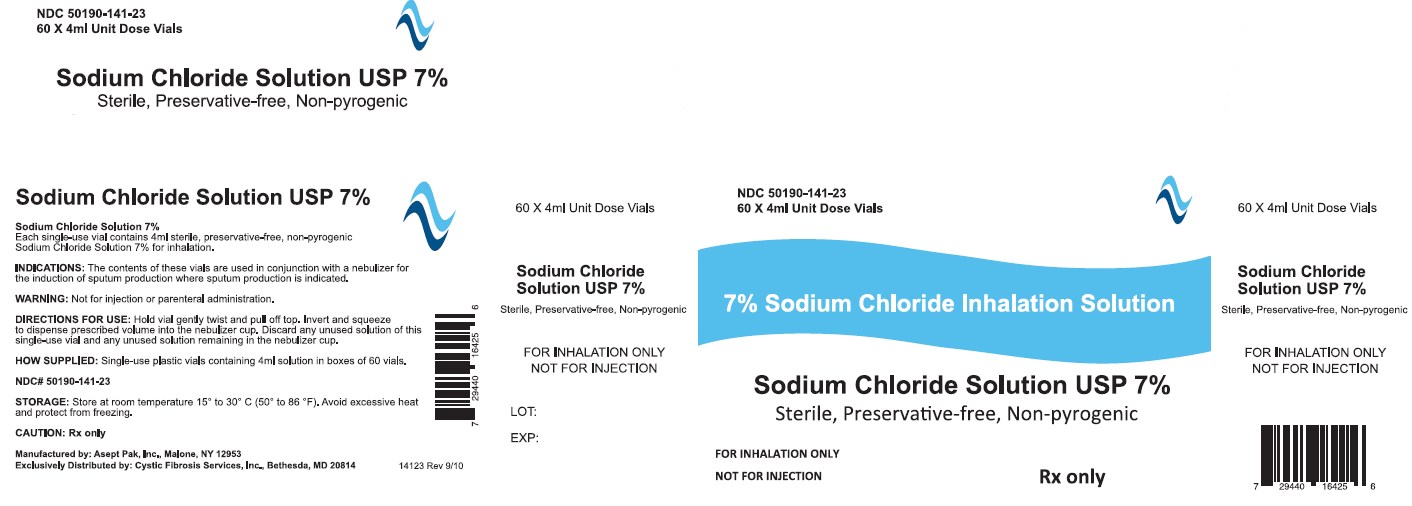
DESCRIPTION
Sodium Chloride Inhalation Solution USP 7% 4 mL
For Respiratory Therapy
Not for parenteral administration.
4 mL, 60 Vials Sterile Unit-Dose Vial
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
Internal contents sterile. External surface of vial not sterile.
INDICATIONS AND USAGE
The contents of these vials are used in conjunction with a nebulizer for the induction of sputum production where sputum production is indicated.
INSTRUCTIONS FOR USE SECTION
Hold vial gently twist and pull off top. Invert and squeeze to dispense prescribed volume into the nebulizer cup.