CVS Iodine Tincture USP
Drug Facts
Active Ingredient
Iodine 2%
Sodium Iodide 2.4%
Alcohol 47%
Indications
To help prevent infection in minor cuts, scrapes, and burns.
Warnings:
For external use only
Ask a doctor before use if you have
Deep or puncture wounds
Animal bites
Serious burns
Stop use and ask a doctor if
the condition persists or gets worse, if using this product for longer than 1 week.
Do not use
in the eyes. If contact occurs, flush with large amounts of water while lifting upper and lower lids.
Do not apply
over large areas of the body.
Keep out of reach of children.
- In case of accidental ingestion. Give milk, then give a starch solution made by mixing two tablespoofuls of cornstarch or flour to a pint of water. Contact a Poison Control Center immediately.
Directions
- Clean the affected area; apply a small amount on the area 1 to 3 times daily; may be covered with a sterile bandage. If bandaged, let dry first.
Inactive Ingredient
Purified water.
Other Information
Will stain skin and clothing
PRINCIPAL DISPLAY PANEL

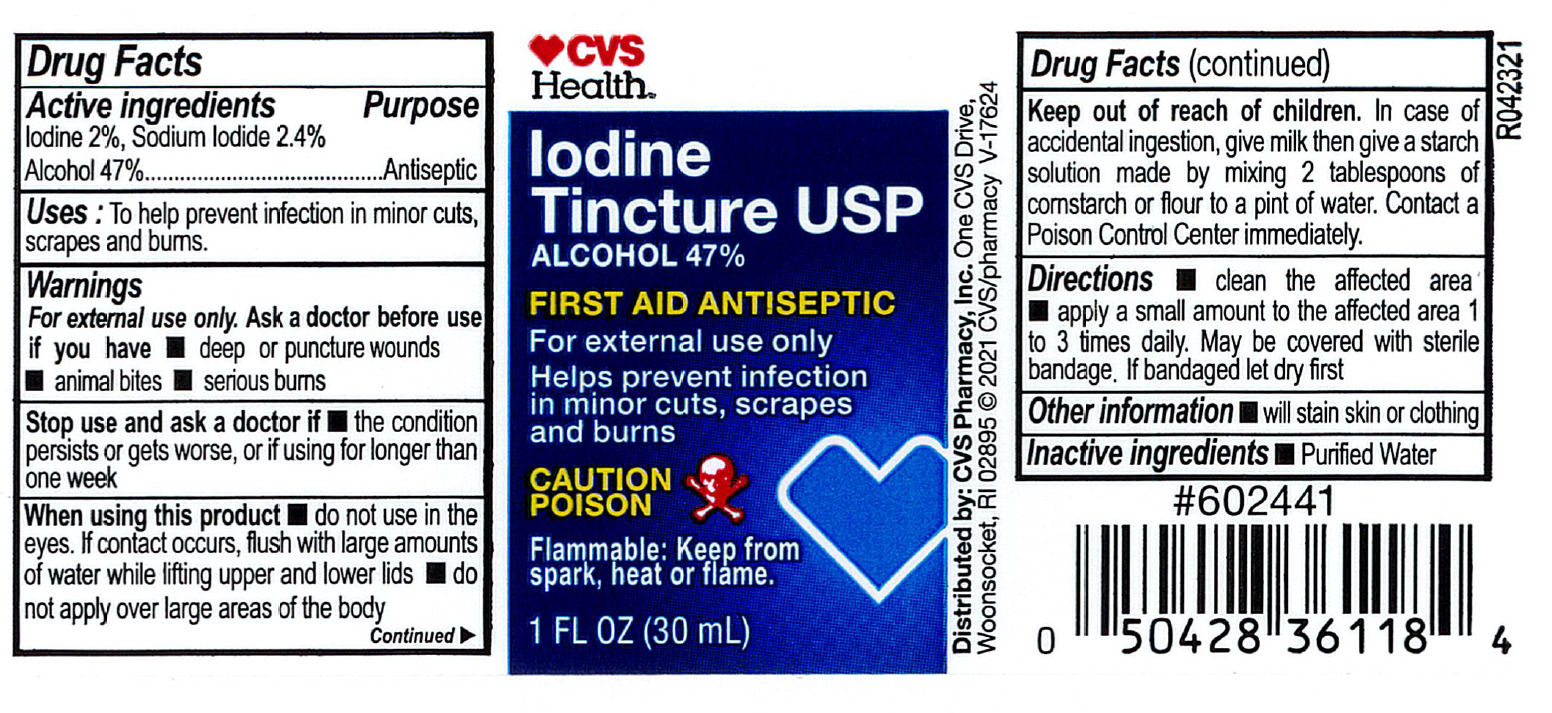 CVS Health
CVS Health
Iodine Tincture USP
First Aid Antiseptic
1 FL OZ (30 mL)
