TC MAX- calcium carbonate powder, for solution
TC MAX- calcium carbonate
Romavision Investing LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
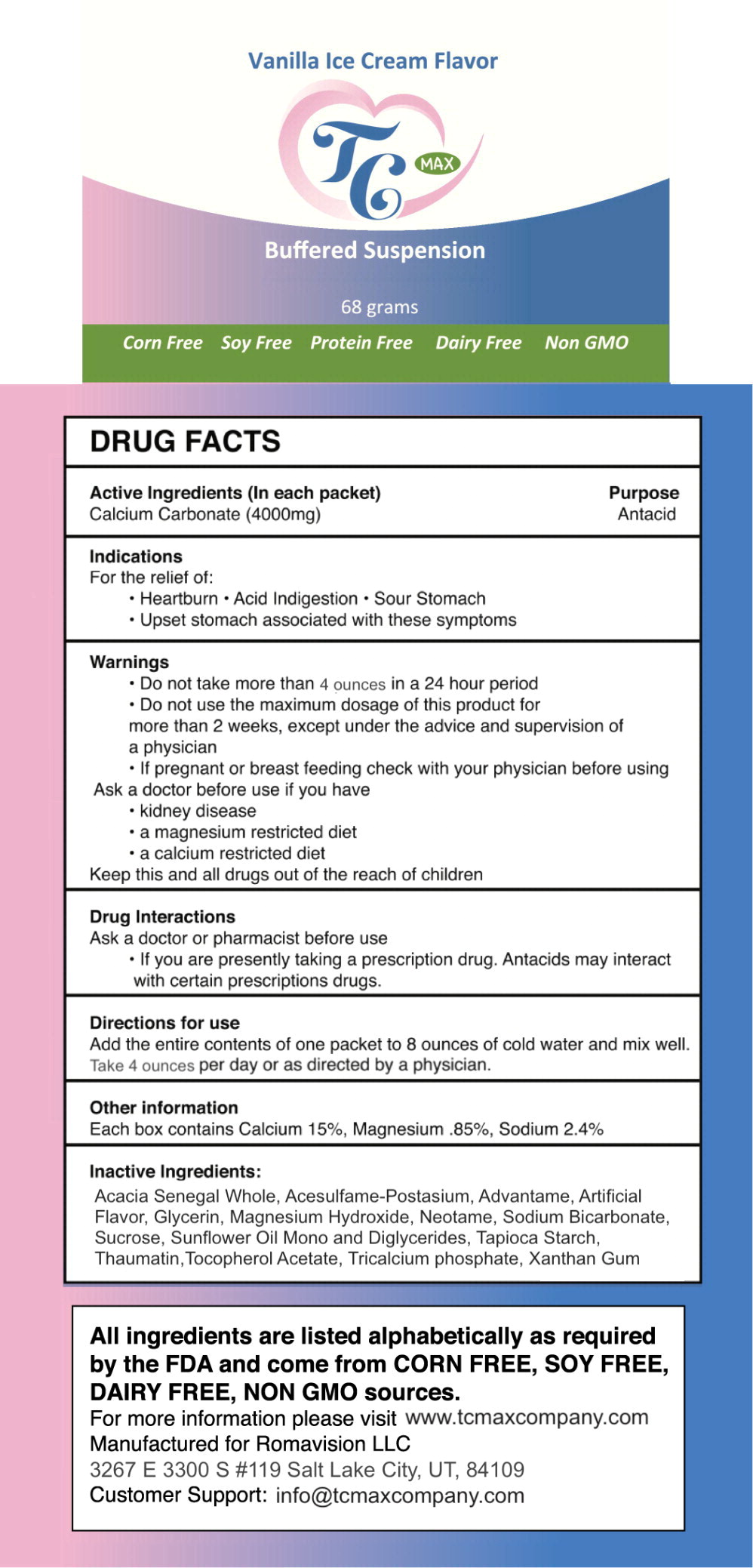
Active Ingredients (In each packet)
Calcium Carbonate (4000mg)
Indications
For the relief of:
- Heartburn
- Acid Indigestion
- Sour Stomach
- Upset stomach associated with these symptoms
Warnings
- Do not take more than 4 ounces in a 24 hour period
- Do not use the maximum dosage of this product for more than 2 weeks, except under the advice and supervision of a physician
- If pregnant or breast feeding check with your physician before using
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- a calcium restricted diet
Keep this and all drugs out of the reach of children
Drug Interactions
Ask a doctor or pharmacist before use
- If you are presently taking a prescription drug. Antacids may interact with certain prescriptions drugs.
Directions for use
Add the entire contents of one packet to 8 ounces of cold water and mix well. Take 4 ounces once per day or as directed by a physician.
Other information
Each box contains Calcium 15%, Magnesium .85%, Sodium 2.4%
Inactive Ingredients:
Acacia Senegal Whole, Acesulfame-Postasium, Advantame, Artificial Flavor, Glycerin, Magnesium Hydroxide, Neotame, Sodium Bicarbonate, Sucrose, Sunflower Oil Mono and Diglycerides, Tapioca Starch, Thaumatin, Tocopherol Acetate, Tricalcium phosphate, Xanthan Gum
Principal Display Panel - 68 gram Packet Label
Vanilla Ice Cream Flavor
TC MAX
Buffered Suspension
68 grams
Corn Free Soy Free Protein Free Dairy Free Non GMO
Principal Display Panel - 68 gram Packet Label
Vanilla Ice Cream
Flavor
TC MAX
Starter Kit
Buffered Suspension
Corn Free Soy Free Protein Free Dairy Free Non GMO