DESCRIPTION
Benzonatate, a non-narcotic oral antitussive agent, is 2, 5, 8, 11, 14, 17, 20, 23, 26-nonaoxaoctacosan-28-yl p-(butylamino) benzoate; with a molecular weight of 603.7.
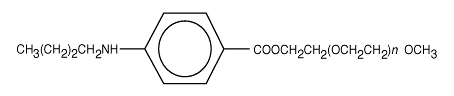
C 30H 53NO 11
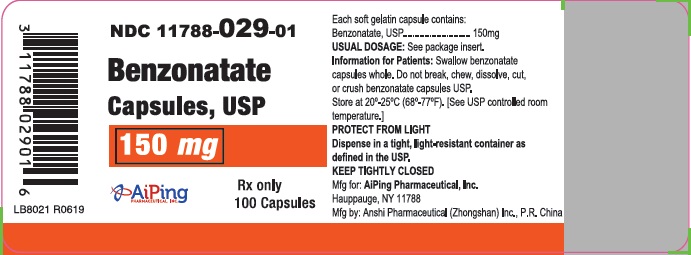
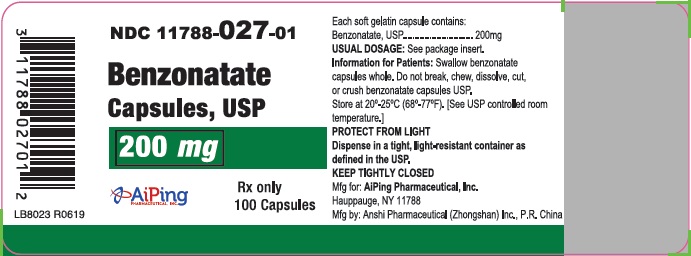
Each soft gelatin capsule, for oral administration, contains 100 mg, 150 mg or 200 mg of benzonatate USP. Benzonatate Capsules, USP also contain the following inactive ingredients: D&C Yellow #10, gelatin, glycerin, purified water, methylparaben and propylparaben. Imprinting ink is composed of isopropyl alcohol, n-butyl alcohol, propylene glycol, shellac, and titanium dioxide.
CLINICAL PHARMACOLOGY
Benzonatate acts peripherally by anesthetizing the stretch receptors located in the respiratory passages, lungs, and pleura by dampening their activity and thereby reducing the cough reflex at its source. It begins to act within 15 to 20 minutes and its effect lasts for 3 to 8 hours. Benzonatate has no inhibitory effect on the respiratory center in recommended dosage.
WARNINGS
Hypersensitivity
Severe hypersensitivity reactions (including bronchospasm, laryngospasm and cardiovascular collapse) have been reported which are possibly related to local anesthesia from sucking or chewing the capsule instead of swallowing it. Severe reactions have required intervention with vasopressor agents and supportive measures.
Psychiatric Efects
Isolated instances of bizarre behavior, including mental confusion and visual hallucinations, have also been reported in patients taking benzonatate in combination with other prescribed drugs.
Accidental Ingestion and Death in Children
Keep benzonatate capsules out of reach of children. Accidental ingestion of benzonatate resulting in death has been reported in children below age 10. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. If accidental ingestion occurs, seek medical attention immediately (see
OVERDOSAGE).
PRECAUTIONS
Benzonatate is chemically related to anesthetic agents of the para-amino-benzoic acid class (e.g. procaine; tetracaine) and has been associated with adverse CNS effects possibly related to a prior sensitivity to related agents or interaction with concomitant medication.
Information for Patients Swallow benzonatate capsules whole. Do not break, chew, dissolve, cut, or crush Benzonatate Capsules. Release of benzonatate from the capsule in the mouth can produce a temporary local anesthesia of the oral mucosa and choking could occur. If numbness or tingling of the tongue, mouth, throat, or face occurs, refrain from oral ingestion of food or liquids until the numbness has resolved. If the symptoms worsen or persist, seek medical attention.
Keep benzonatate out of reach of children. Accidental ingestion resulting in death has been reported in children. Signs and symptoms of overdose have been reported within 15-20 minutes and death has been reported within one hour of ingestion. Signs and symptoms may include restlessness, tremors, convulsions, coma and cardiac arrest. If accidental ingestion occurs, seek medical attention immediately.
Overdosage resulting in death may occur in adults.
Do not exceed a single dose of 200 mg and a total daily dosage of 600 mg. If you miss a dose of benzonatate capsule, skip that dose and take the next dose at the next scheduled time. Do not take 2 doses of benzonatate at one time.
Usage in Pregnancy
PREGNANCY CATEGORY C
Animal reproduction studies have not been conducted with benzonatate. It is also not known whether benzonatate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzonatate should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk caution should be exercised when benzonatate is administered to a nursing woman.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and reproduction studies have not been conducted with benzonatate.
Pediatric Use
Safety and effectiveness in children below the age of 10 have not been established. Accidental ingestion resulting in death has been reported in children below age 10. Keep out of reach of children.
ADVERSE REACTIONS
Potential Adverse Reactions to benzonatate may include:
Hypersensitivity reactions including bronchospasm, laryngospasm, cardiovascular collapse possibly related to local anesthesia from chewing or sucking the capsule.
CNS: sedation; headache; dizziness; mental confusion; visual hallucinations.
GI: constipation; nausea; GI upset.
Dermatologic: pruritus; skin eruptions.
Other: nasal congestion; sensation of burning in the eyes; vague “chilly” sensation; numbness of the chest; hypersensitivity. Deliberate or accidental overdose has resulted in death, particularly in children.
OVERDOSAGE
Intentional and unintentional overdose may result in death, particularly in children. The drug is chemically related to tetracaine and other topical anesthetics and shares various aspects of their pharmacology and toxicology. Drugs of this type are generally well absorbed after ingestion.
Signs and Symptoms
The signs and symptoms of overdose of benzonatate have been reported within 15-20 minutes. If capsules are chewed or dissolved in the mouth, oropharyngeal anesthesia will develop rapidly, which may cause choking and airway compromise. CNS stimulation may cause restlessness and tremors which may proceed to clonic convulsions followed by profound CNS depression. Convulsions, coma, cerebral edema and cardiac arrest leading to death have been reported within 1 hour of ingestion.
Treatment
In case of overdose, seek medical attention immediately. Evacuate gastric contents and administer copious amounts of activated charcoal slurry. Even in the conscious patient, cough and gag reflexes may be so depressed as to necessitate special attention to protection against aspiration of gastric contents and orally administered materials. Convulsions should be treated with a short-acting barbiturate given intravenously and carefully titrated for the smallest effective dosage. Intensive support of respiration and cardiovascular-renal function is an essential feature of the treatment of severe intoxication from overdosage. Do not use CNS stimulants.
DOSAGE AND ADMINISTRATION
Adults and Children over 10 years of age: Usual dose is one 100 mg, 150 mg or 200 mg capsule three times a day as needed for cough. If necessary to control cough, up to 600 mg daily in three divided doses may be given.
Benzonatate should be swallowed whole. Benzonatate capsules are not to be broken, chewed, dissolved, cut or crushed.
HOW SUPPLIED
Benzonatate Capsules USP, 100 mg: Yellow soft gelatin capsules, imprinted "
 "with white ink, available in bottles of 100’s (NDC 11788-028-01), and 500’s (NDC 11788-028-05).
"with white ink, available in bottles of 100’s (NDC 11788-028-01), and 500’s (NDC 11788-028-05).
Benzonatate Capsules USP, 150 mg: Yellow soft gelatin capsules, imprinted "
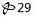 "with white ink, available in bottles of 100's (NDC 11788-029-01), and 500’s (NDC 11788-029-05).
"with white ink, available in bottles of 100's (NDC 11788-029-01), and 500’s (NDC 11788-029-05).
Benzonatate Capsules USP, 200 mg: Yellow soft gelatin capsules, imprinted "
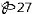 "with white ink, available in bottles of 100’s (NDC 11788-027-01) and 500’s (NDC 11788-027-05).
"with white ink, available in bottles of 100’s (NDC 11788-027-01) and 500’s (NDC 11788-027-05).
Store at 20° to 25° C (68° to 77°F). [See USP Controlled Room Temperature]. PROTECT FROM LIGHT. Dispense in a tight, light-resistant container as defined in the USP.
Manufactured for
AiPing Pharmaceutical, Inc.
Hauppauge, NY 11788 USA
Manufactured by
Anshi Pharmaceutical (Zhongshan) Inc., P.R. China
Rev 03/18