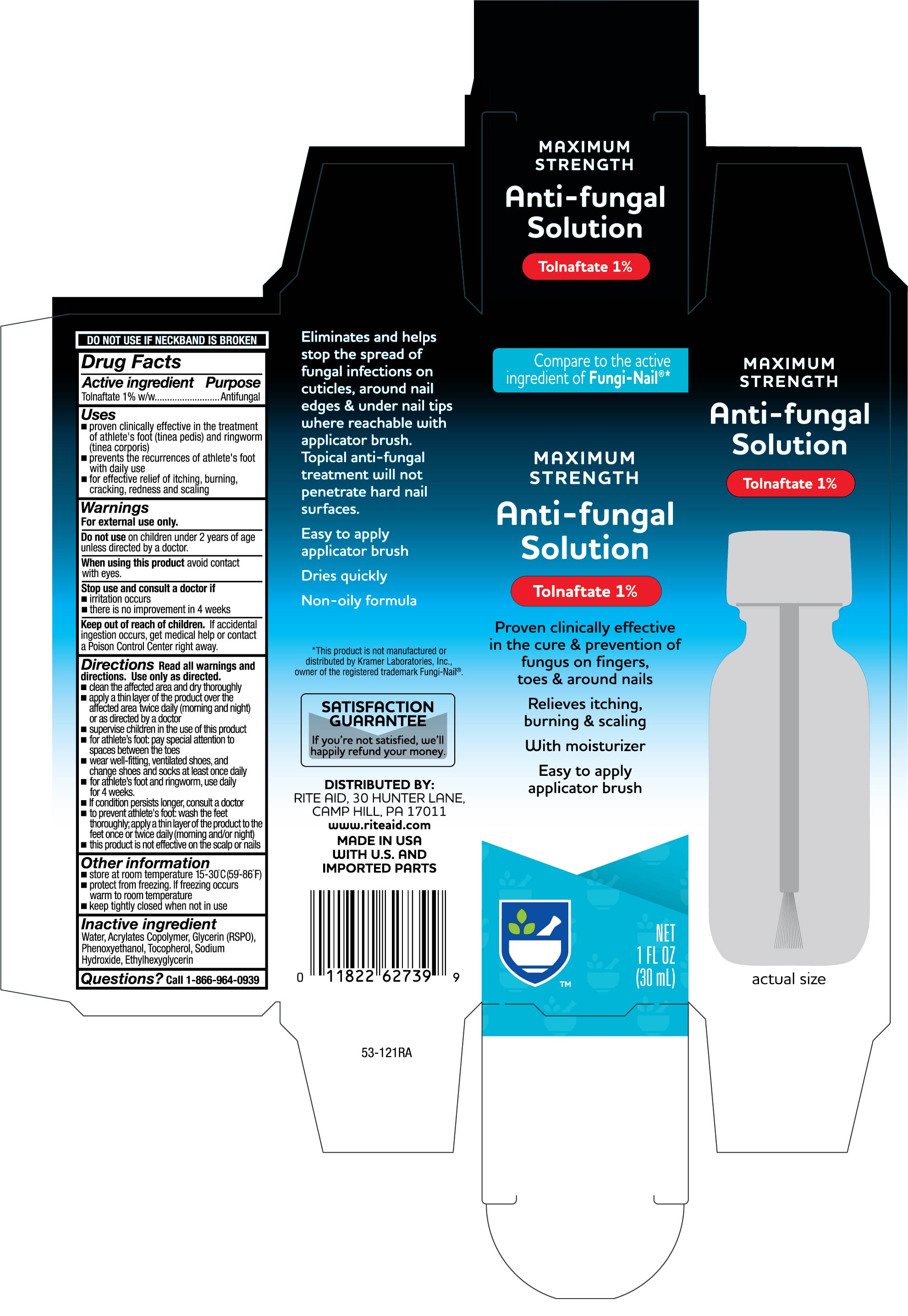
Uses
proven clinically effective in the treatment of athlete's foot (tinea pedis) and ringworm (tinea corportis)
prevents the recurrences of athlete's foot with daily use
for effective relief of itching, burning, cracking, redness and scaling
Directions
Read all warnings and directions. Use only as directed.
- clean the affected area and dry thoroughly
- apply a thin layer of the product over the affected area twice daily (morning and night) or as directed by a doctor
- supervise children in the use of this product
- for athlete's foot: pay special attention to spaces between toes
- wear well-fitting, ventilated shoes, and change shoes and socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks.
- If condition persists longer, consult a doctor
- to prevent athlete's foot: wash the feet thoroughly, apply a thin layer of the product to the feet once or twice daily (morning and/or night)
- this product is not effective on the scalp or nails
Other information
- store at room temperature 15°-30°C (59° - 86°F)
- protect from freezing. If freezing occurs warm to room temperature
- keep tightly closed when not in use