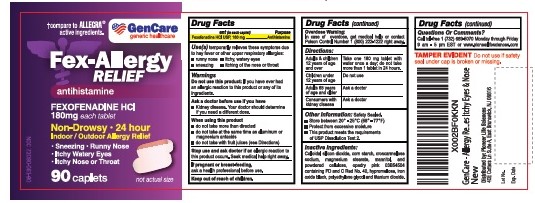
Uses:
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Do not use:
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients.
WHEN USING THIS PRODUCT
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
ASK A DOCTOR BEFORE USE IF
Kidney disease. Your doctor should determine if you need a different dose.
STOP USE AND ASK A DOCTOR IF
an allergic reaction to this product occurs. Seek medical help right away.
KEEP OUT OF REACH OF CHILDREN
In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS
| adults and children 12 years of age and over | Take one 180 mg tablet with water once a day; do not take more than 1 tablets in 24 hours. |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
OTHER INFORMATION
- store at 20°-25°C (68°-77°F)
- protect from excessive moisture
- this product meets the requirement of USP dissolution test 2.
INACTIVE INGREDIENTS
colloidal silicon dioxide, corn starch,croscarmellose sodium, magnesium stearate,mannitol and microcrystalline cellulose, opadry pink 03B54504 containing FD and C red No.40, iron oxide black,hypromellose,polyethylene glycol, titanium dioxide.
QUESTIONS OR COMMENTS?
Call 1-732-698-5070 Monday through Friday 9AM–5PM EST or www.pioneerlifesciences.com