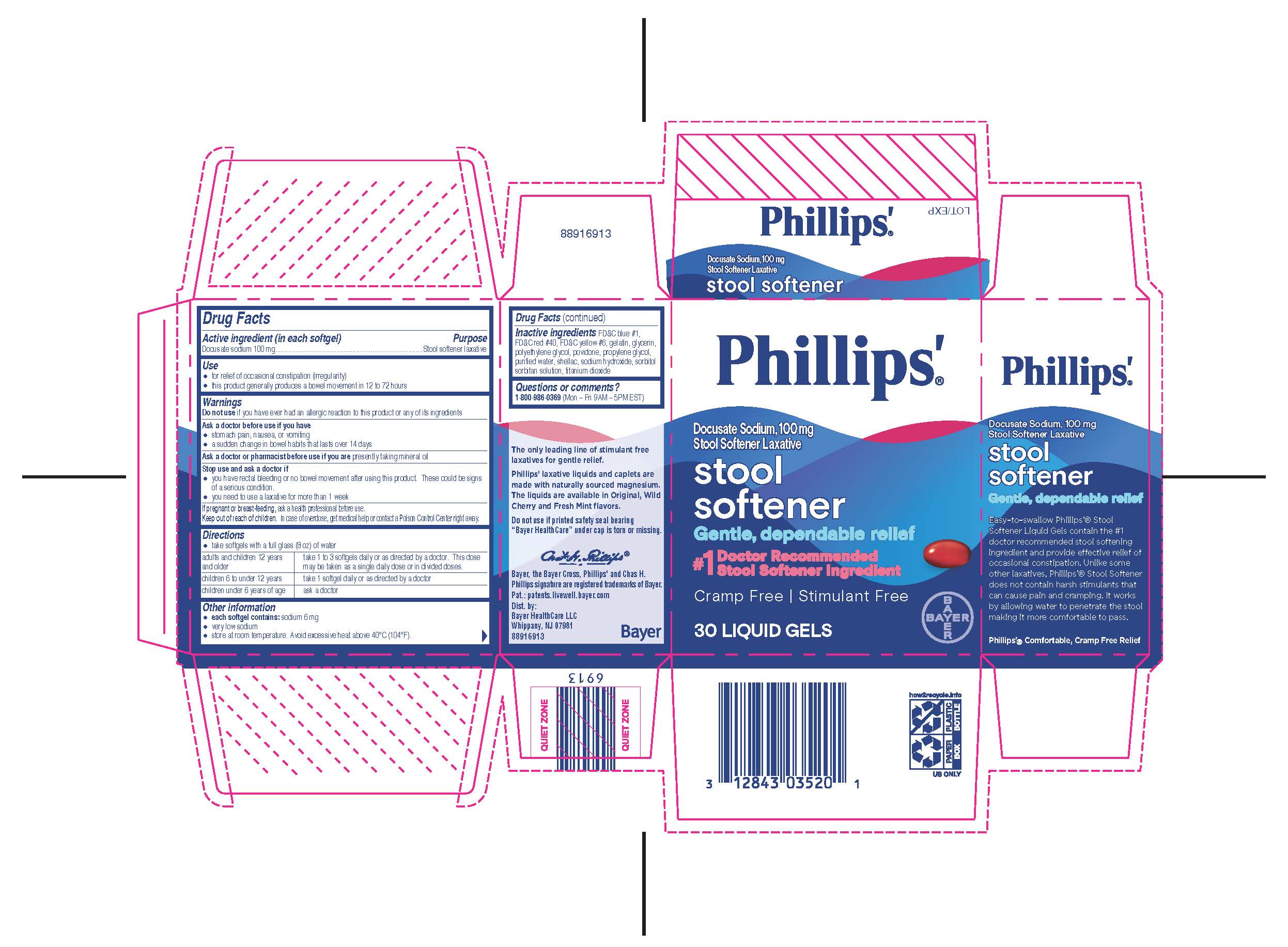
PHILLIPS STOOL SOFTENER BULK DOCUSATE- docusate sodium capsule, liquid filled
Bayer HealthCare LLC.
----------
Phillips ®' Stool Softener Liquid Gels
Use
- for relief of occasional constipation (irregularity)
- this product generally produces a bowel movement in 12 to 72 hours
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients.
Ask a doctor before use if you have
- stomach pain, nausea, or vomiting
- a sudden change in bowel habits that lasts over 14 days
Directions
- take softgels with a full glass (8 oz) of water
| adults and children 12 years and older | take 1 to 3 softgels daily or as directed by a doctor. This dose may be taken as a single daily dose or in divided doses. |
| children 6 to under 12 years | take 1 softgel daily or as directed by a doctor |
| children under 6 years of age | ask a doctor |
Other information
- each softgel contains: sodium 6 mg
- very low sodium
- store at room temperature. Avoid excessive heat 40°C (104°F).
| PHILLIPS STOOL SOFTENER
BULK DOCUSATE
docusate sodium capsule, liquid filled |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |
Revised: 11/2023
Document Id: 0b61f06f-ec5c-c6db-e063-6394a90a724e
Set id: b0462555-5554-4a17-a1fe-4206a4fd350d
Version: 12
Effective Time: 20231130
Bayer HealthCare LLC.