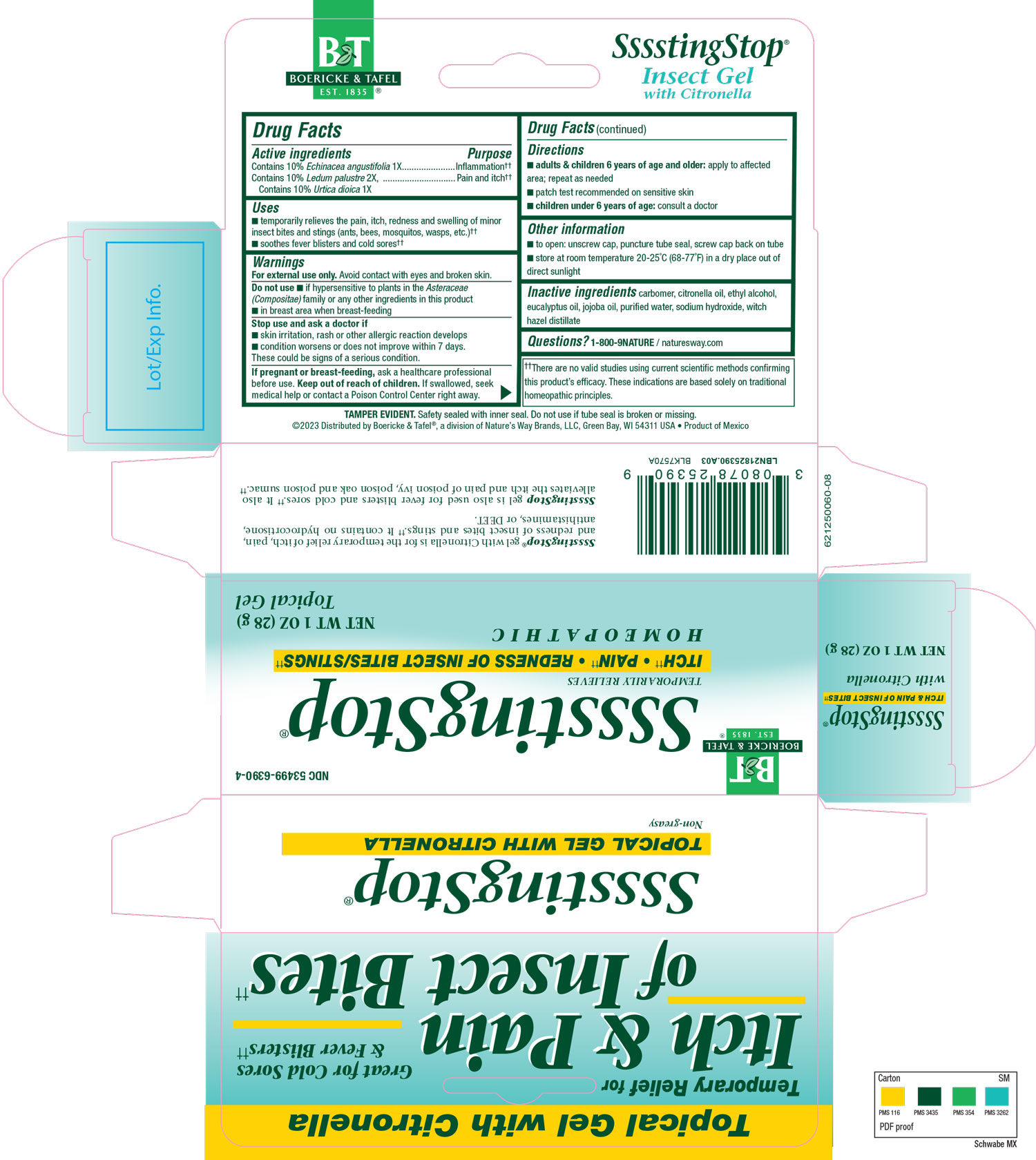
Inactive Ingredient
carbomer
citronella oil
ethyl alcohol
eucalyptus oil
jojoba oil
purified water
sodium hydroxide
witch hazel distillate
Indications & Usage
Temporarily relieves the pain, itch, redness and swelling of minor insect bites and stings (ants, bees, mosquitos, wasps, etc).
Soothes fever blisters and cold sores.
Purpose
Temporarily relieves the pain, itch, redness and swelling of minor insect bites and stings (ants, bees, mosquitos, wasps, etc).
Soothes fever blisters and cold sores.
Dosage & Administration
Directions:
Adults & children 6 years of age and older: Apply to affected area; repeat as needed.
Patch test recommended on sensitive skin.
Children under 6 years of age: consult a doctor.
Do not use
Do not use if hypersensitive to plants in the Asteraceae (Compositae) family or any other ingredients in this product, in breast area when breast-feeding.
Stop Use
Stop use and ask a doctor if skin irritation, rash or other allergic reaction develops, condition worsens or does not improve within 7 days.
These could be signs of a serious condition.