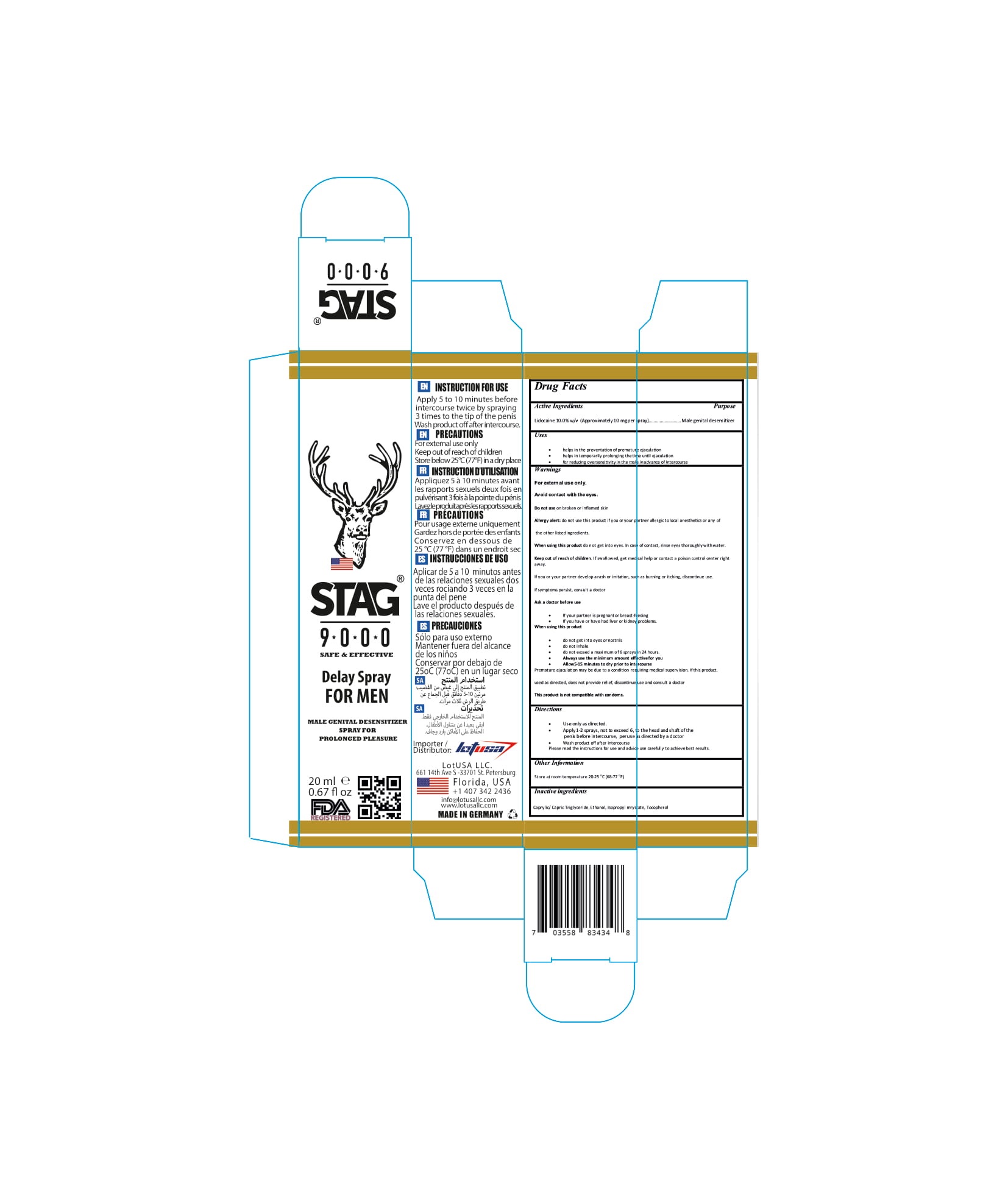
- Lidocaine 10.0% w/v (Approximately10 mgper spray)
- Caprylic/ Capric Triglyceride,
- Ethanol,
- Isopropyl mrystate,
- Tocopherol
The firm does not add other active or inactive ingredients. Different or additional ingredients may impact the quality and potency of the product.
Active Ingredient(s)
Lidocaine 10.0% w/v (Approximately10 mgper spray) Purpose: Male genital desensitizer
Use
• helps in the preventation of premature ejaculation
• helps in temporarily prolonging thetime until ejaculation
• for reducing oversensitivity in the male inadvance of intercourse
When using this product do not get into eyes. In case of contact, rinse eyes thoroughly withwater.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
If you or your partner develop a rash or irritation, such as burning or itching, discontinue use. Ifsymptoms persist, consult a doctor Ask a doctor before use
If your partner is pregnant or breast-feeding
If you have or have had liver or kidney problems. When using this pr oduct
do not get into eyes or nostrils
do not inhale
do not exceed a maximum of 6 sprays in 24 hours.
Always use the minimum amount effectivefor you
Allow5-15 minutes to dry prior to intercourse Premature ejaculation may be due to a condition requiring medical supervision. If this product, used as directed, does not provide relief, discontinue use and consult a doctor This product is not compatible with condoms.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.