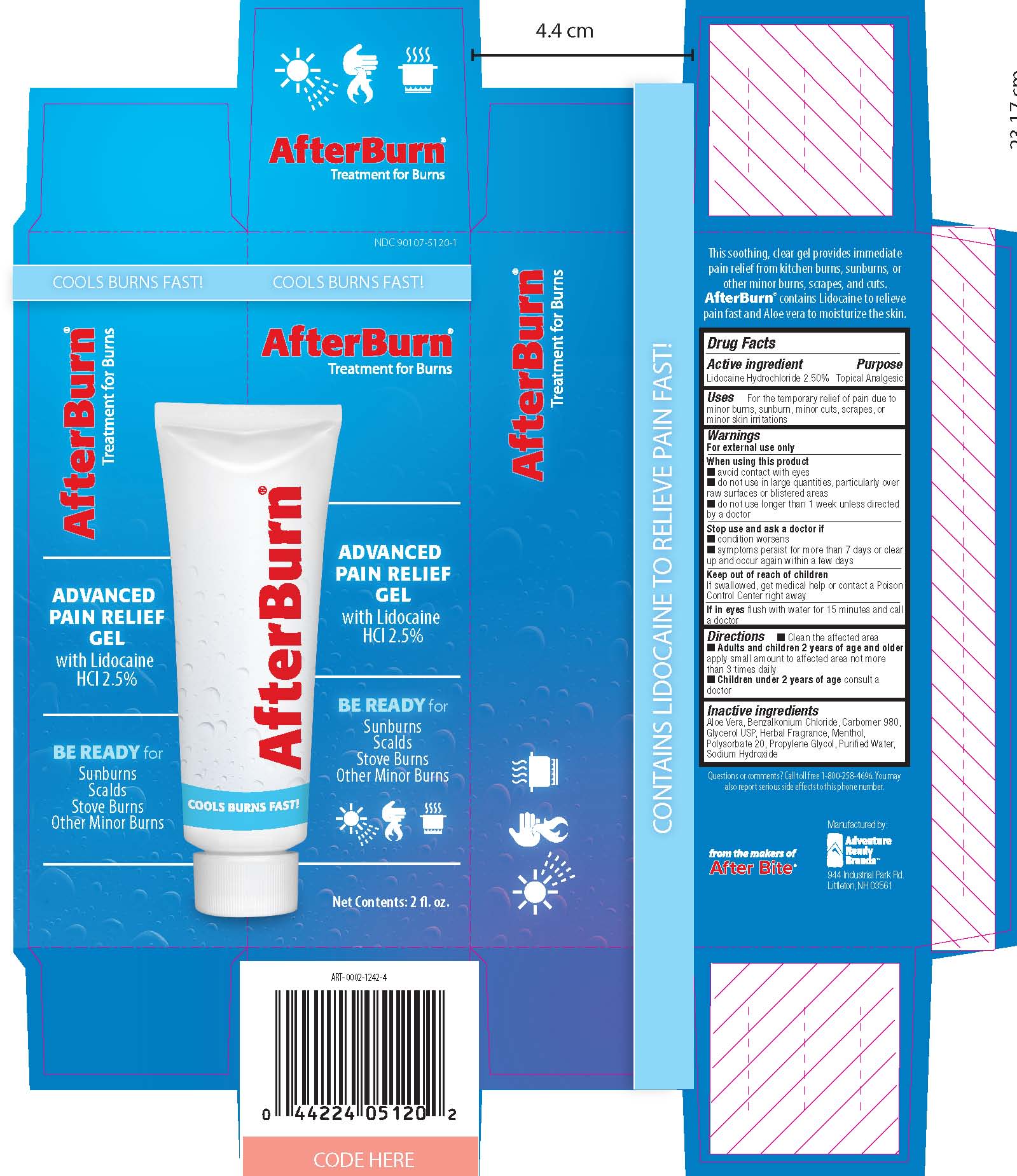
Uses
For the temporary relief of pain due to minor burns, sunburn, minor cuts, screapes or minor skin irritations
When using this product
- avoid contact with eyes
- do not use in large quantities, particularly over raw surfaces or blistered areas
- do not use longer than 1 week unless directed by a doctor
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
If in eyes, flush with water for 15 minutes and call a doctor.
Directions
Clean affected area
Adults and Children 2 years and older apply small amount to affected area not more than 3 times daily
Children under 2 years consult a doctor.