This is a hand sanitizer manufactured according to the Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (CoViD-19); Guidance for Industry.
The hand sanitizer is manufactured using only the following United States Pharmacopoeia (USP) grade ingredients in the preparation of the product (percentage in final product formulation) consistent with World Health Organization (WHO) recommendations:
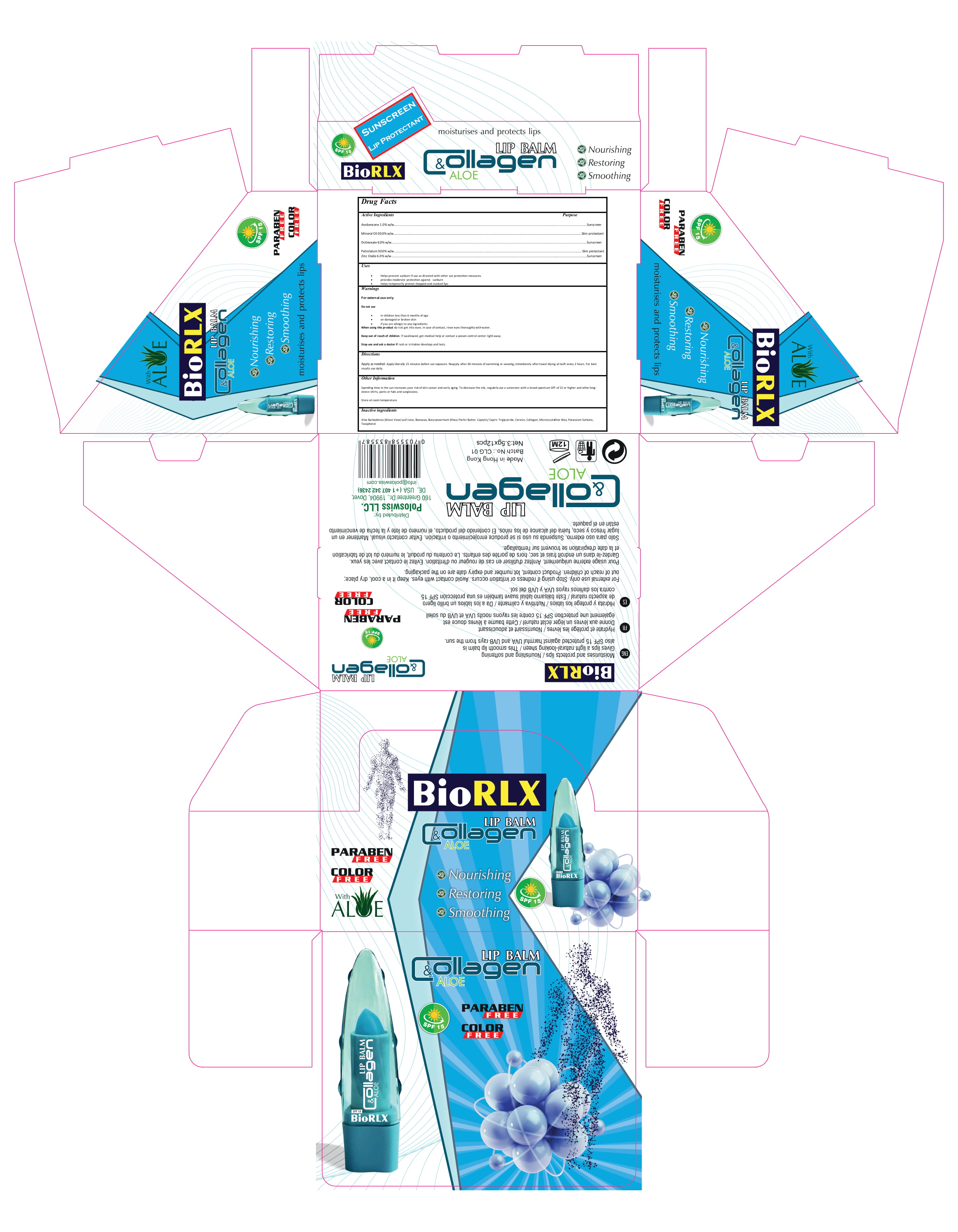
a. Avobenzone 1.0%w/w
b. Mineral Oil 30.0%w/w
c. Octinoxate 6.0% w/w
d. Petrolatum 30.0% w/w
e. Zinc Oxide 6.0% w/w
f. Aloe Barbadensis (Alove Vera) Leaf Juice
g. Beeswax
h. Butyrpsoermum (Shea) Parkii Butter
i.Caprylic/ Capric Triglyceride
j. Ceresin
k.Collagen
l. Microcrystalline Wax
m. Potassium Sorbate
n. Tocopherol
The firm does not add other active or inactive ingredients. Different or additional ingredients may impact the quality and potency of the product.
Active Ingredient(s)
Avobenzone 1.0% w/w Sunscreen
Mineral Oil 30.0% w/w Skin protectant
Octinoxate 6.0% w/w Sunscreen
Petrolatum 30.0% w/w Skin protectant
Zinc Oxide 6.0% w/w Sunscreen
Use
Helps prevent sunburn if use as directed with other sun protection measures.
provides moderate protection against sunburn
Helps temporarily protect chapped and cracked lips.
Do not use
in children less than 6 months of age on damaged or broken skin if you are allergic to any ingredients
When using this product keep out of eyes, ears, and mouth. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use and ask a doctor if irritation or rash occurs. These may be signs of a serious condition.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Apply as needed. Apply liberally 15 minutes before sun exposure. Reapply after 60 minutes of swimming or sweatig, immediately after towel drying at leaft every 2 hours. For best results use daily.
Other information
- Spending time in the sun increases your risk of skin cancer and early aging. To decrease the risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other longsleeve shirts, pants or hats and sunglassess.
Store at room temperature