BISACODYL - bisacodyl suppository
CARDINAL HEALTH, INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
ACTIVE INGREDIENT (in each suppository)
Bisacodyl USP, 10 mg
USES
For the relief of occasional constipation. Bowel movement is generally produced in 15 minutes to 1 hour.
WARNINGS
For rectal use only
Do not use
laxative products for a period longer than one week unless directed by a doctor
Ask a doctor before use if you have
• abdominal pain, nausea or vomiting
• a sudden change in bowel habits that lasts longer than 2 weeks
Stop use and ask a doctor
if rectal bleeding occurs or you fail to have a bowel movement after using a laxative. This may indicate a serious condition.
IF PREGNANT OR BREAST FEEDING,
ask a health professional before use.
KEEP OUT OF REACH OF CHILDREN SECTION
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
• Remove foil. Insert suppository well into rectum touching the bowel wall. Retain about 15 to 20 minutes.
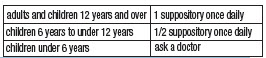
• In the presence of anal fissures or hemorrhoids, suppositories should be coated at the tip with petroleum jelly.
OTHER INFORMATION
Store at room temperature
INACTIVE INGREDIENT
hydrogenated vegetable oil
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 37205-102-53
LEADER®
BISACODYL SUPPOSITORIES
LAXATIVE
FOR PROMPT RELIEF OF CONSTIPATION
12 BISACODYL SUPPOSITORIES 10 MG EACH
Compare to Dulcolax® active ingredient*
Satisfaction Guaranteed
CARDINAL HEALTH, INC.

