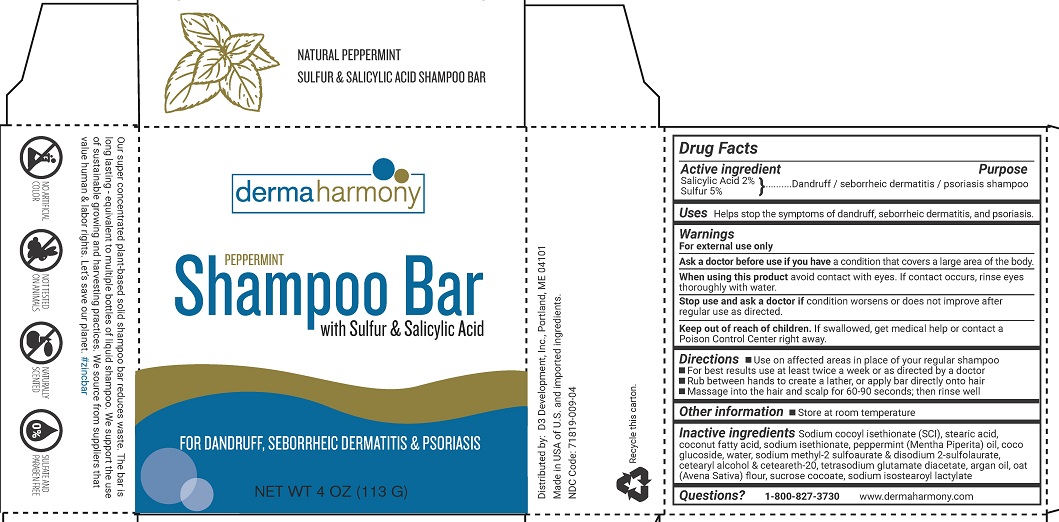
DERMAHARMONY BAR PEPPERMINT- salicylic acid, sulfur shampoo
D3 Development, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Salicylic Acid 2%
Sulfur 5%
Purpose
Dandruff / sebhorreic dermatitis / psoriasis shampoo
Uses
Helps stop the symptoms of dandruff, sebhorreic dermatitis, and psoriasis.
Warnings
For external use only
Ask a doctor before use if you have a condition that covers a large area of the body.
When using this product, avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask a doctor if condition worsens or does not improve after regular use as directed.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- Use on affected areas in place of your regular shampoo
- For best results use at least twice a week or as directed by a doctor
- Rub between hands to create a lather, or apply bar directly onto hair
- Massage into the hair and scalp for 60-90 seconds; then rinse well
Other information
- Store at room temperature
Inactive ingredients
Sodium cocoyl isethionate (SCI), stearic acid, coconut fatty acid, sodium isethionate, peppermint (Mentha Piperita) oil, coco glucoside, water, sodium methyl 2 sulfolaurate & disodium-2-sulfolaurate, cetearyl alcohol & ceteareth-20, tetrasodium glutamate diacetate, argan oil, oat (Avena Sativa) flour, sucrose cocoate, sodium isostearoyl lactylate
Questions?
1-800-827-3730
www.dermaharmony.com
Distributed by:
D3 Development, Inc., Portland, ME 04101
Made in USA of U.S. and imported ingredients
NDC 71819-009-04
dermaharmony
PEPPERMINT
Shampoo Bar with Sulfur & Salicylic Acid
FOR DANDRUFF, SEBORRHEIC DERMATITIS & PSORIASIS
NET WT 4 OZ (113 G)
D3 Development, Inc.
