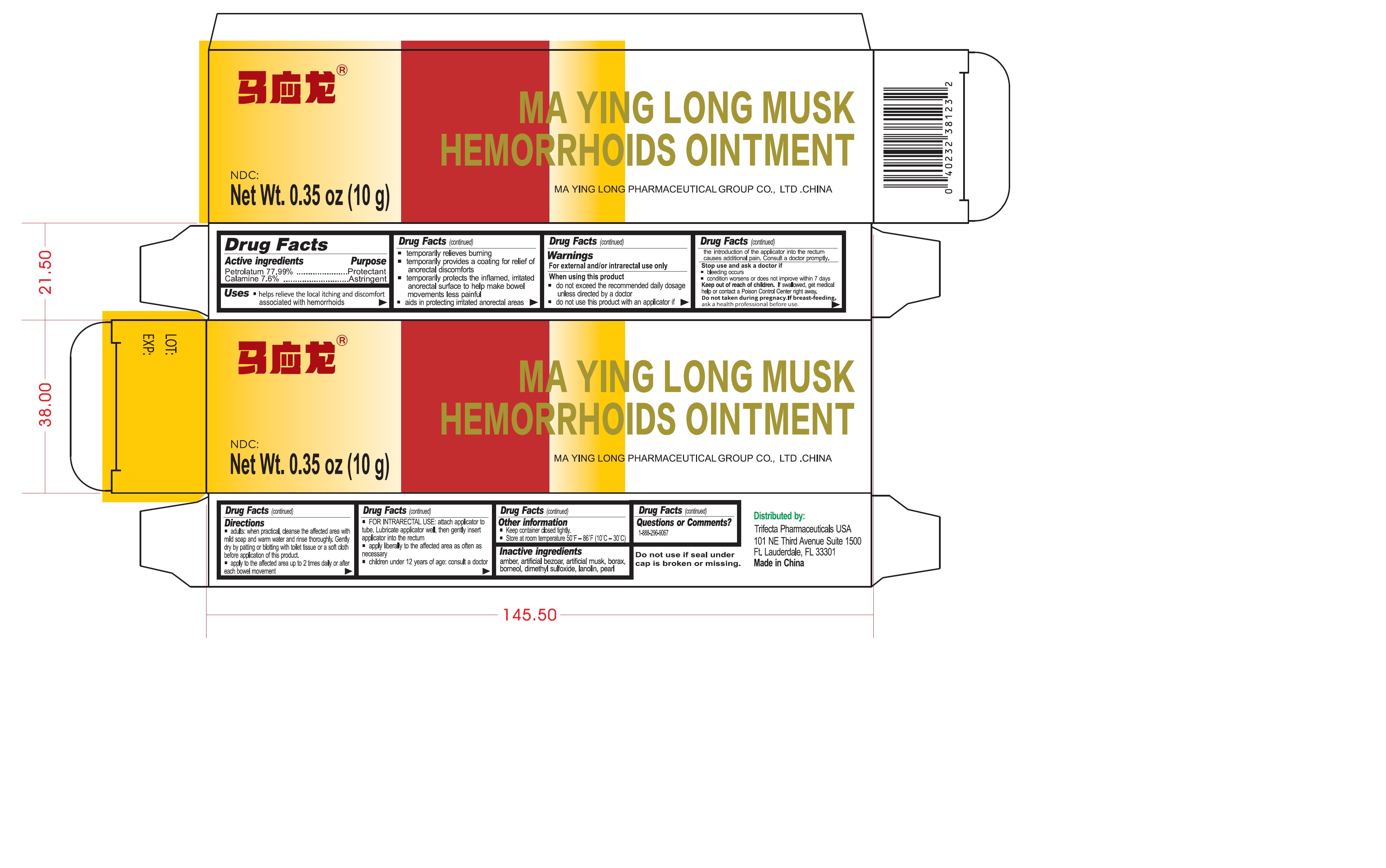
Uses
- helps relieve the local itching and discomfort associated with hemorrhoids
- temporaily relieves burning
- temporarily provides a coating for relief of anorectal discomforts
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
- aids in protecting irritated anorectal areas
Warnings
For external and/or intrarectal use only
When using this product
- do not exceed the recommended daily dosage unless directed by a doctor
- do not use this product with an applicator if the introduction of the applicator into the rectum causes additional pain. Consult a doctor promptly.
Directions
- adults: when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- apply to the affected area up to 2 times daily or after each bowel movement
- FOR INTRARECTAL USE: attach applicator to tube. Lubricant applicator well, then gently insert applicator into the rectum
- apply liberally to the affected area as often as necessary
- children under 12 years of age: consult a doctor