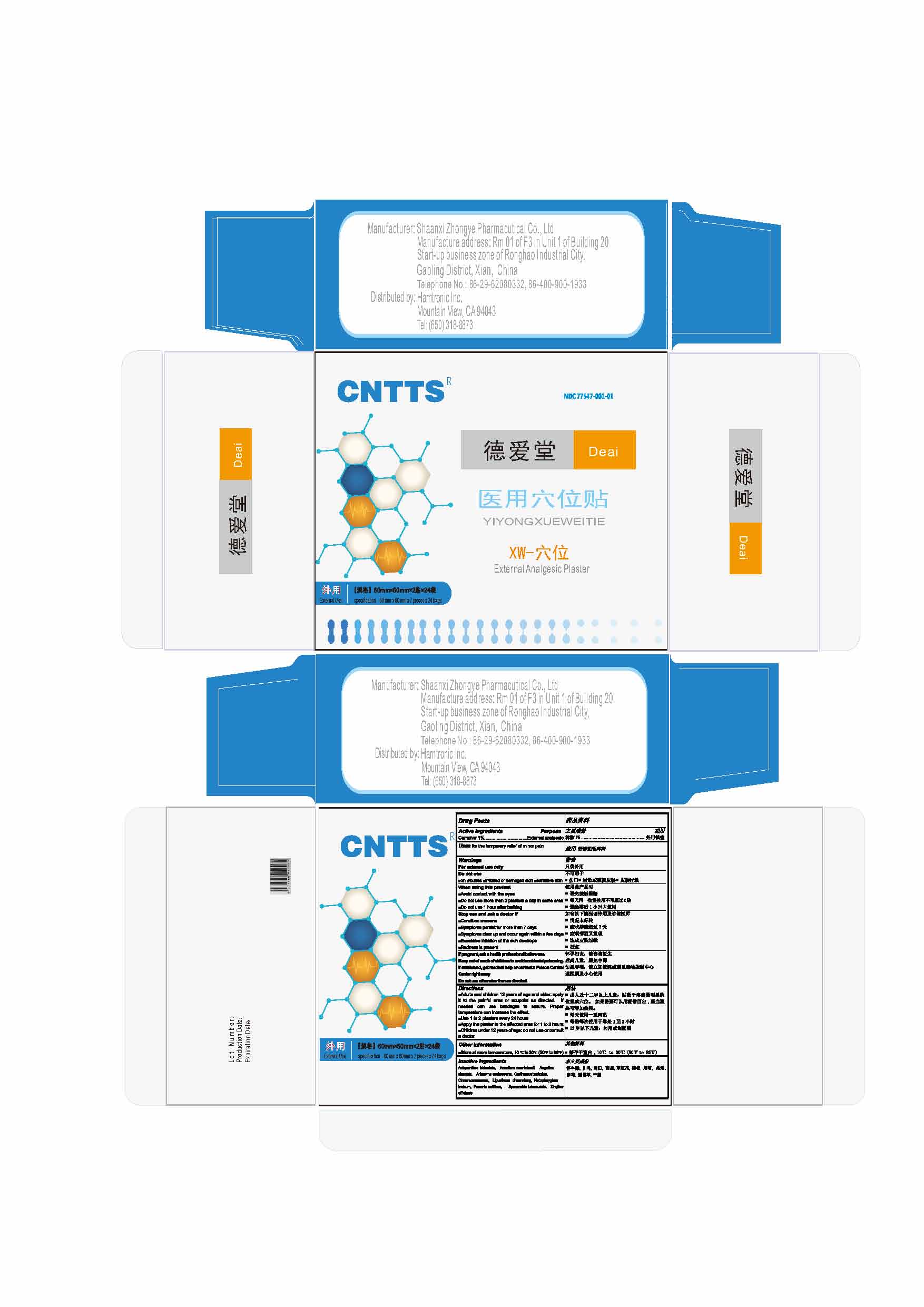
Keep out of reach of children
Keep out of reach of children to avoid accidental poisoning
If swallowed, get medical help or contact a Poison Control Center right away
Directions
■Adults and children 12 years of age and older: apply it to the painful area or acupoint as directed. If needed can use bandages to secure. Proper temperature can increase the effect.
■Use 1 to 2 plasters every 24 hours
■Apply the plaster to the affected area for 1 to 2 hours
■Children under 12 years of age: do not use or consult a doctor.
Inactive ingredients
Achyranthes bidentate, Aconitum carmichaeli, Angelica sinensis, Arisaema erubescens, Carthamus tinctorius, Cinnamomum cassia, Ligusticum chuanxiong, Notopterygium incisum, Paeonia lactiflora, Speranskia tuberculate, Zingiber officinale
When using this product
■Avoid contact with the eyes
■Do not use more than 2 plasters a day in same area
■Do not use 1 hour after bathing