BACTI-FOAM- triclosan solution
Ecolab Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
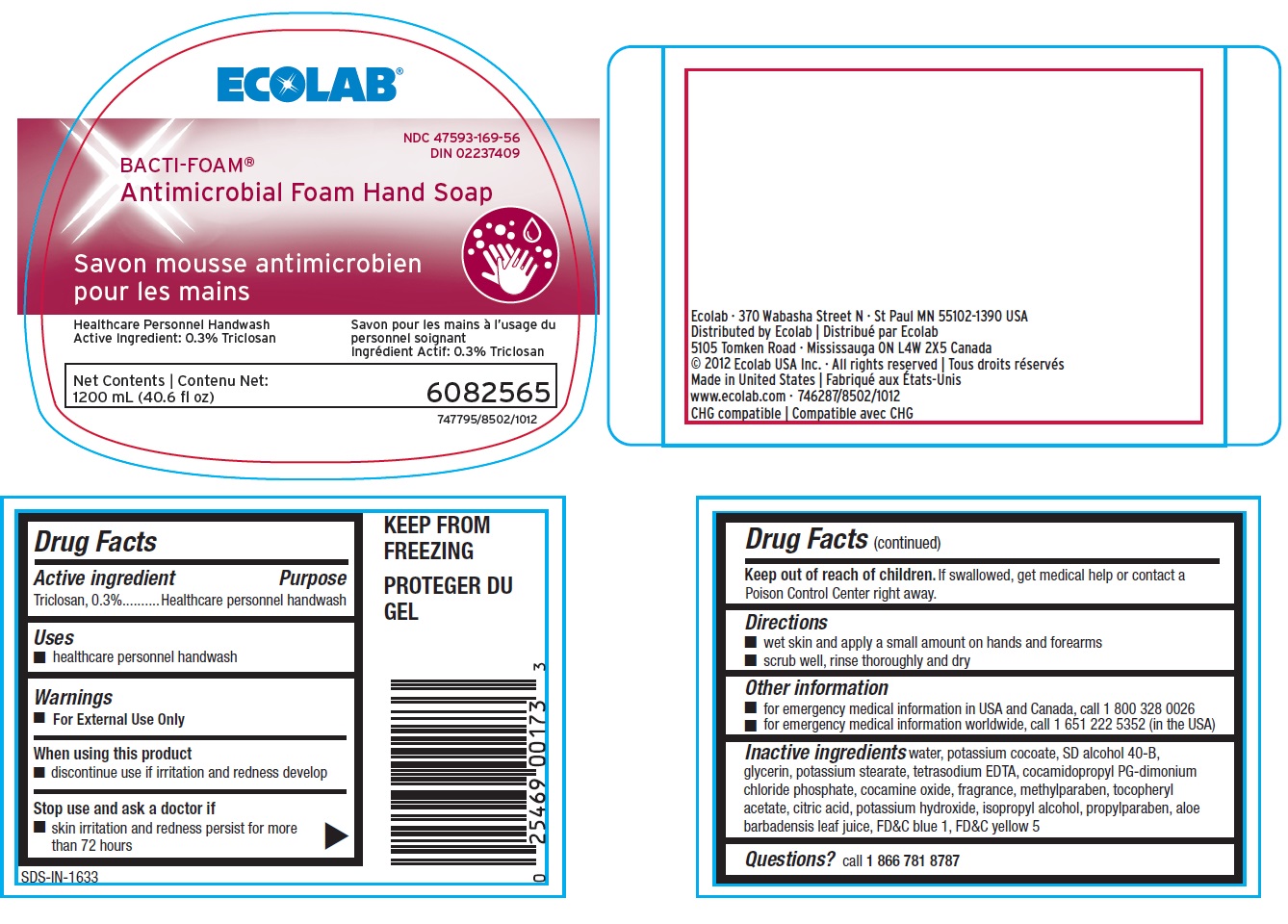
Drug Facts
Directions
- wet skin and apply a small amount on hands and forearms
- scrub well, rinse thoroughly and dry
Other information
- for emergency medical information in USA and Canada, call 1.800.328.0026
- for emergency medical information worldwide, cal 1.651.222.5352 (in the USA)
Inactive ingredients water, potassium cocoate, SD alcohol 40-B, glycerin, potassium stearate, tetrasodium EDTA, cocamidopropyl PG-dimonium chloride phosphate, cocamine oxide, fragrance, methylparaben, tocopheryl acetate, citric acid, potassium hydroxide, isopropyl alcohol, propylparaben, aloe barbadensis leaf juice, FDC blue 1, FDC yellow 5
| BACTI-FOAM
triclosan solution |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Labeler - Ecolab Inc. (006154611) |
Revised: 1/2021
Document Id: af1f22fa-d062-419f-9cf8-69e36fa6da71
Set id: af3a3c04-5d55-46d6-bbf5-db8dfc7b9484
Version: 6
Effective Time: 20210108
Ecolab Inc.