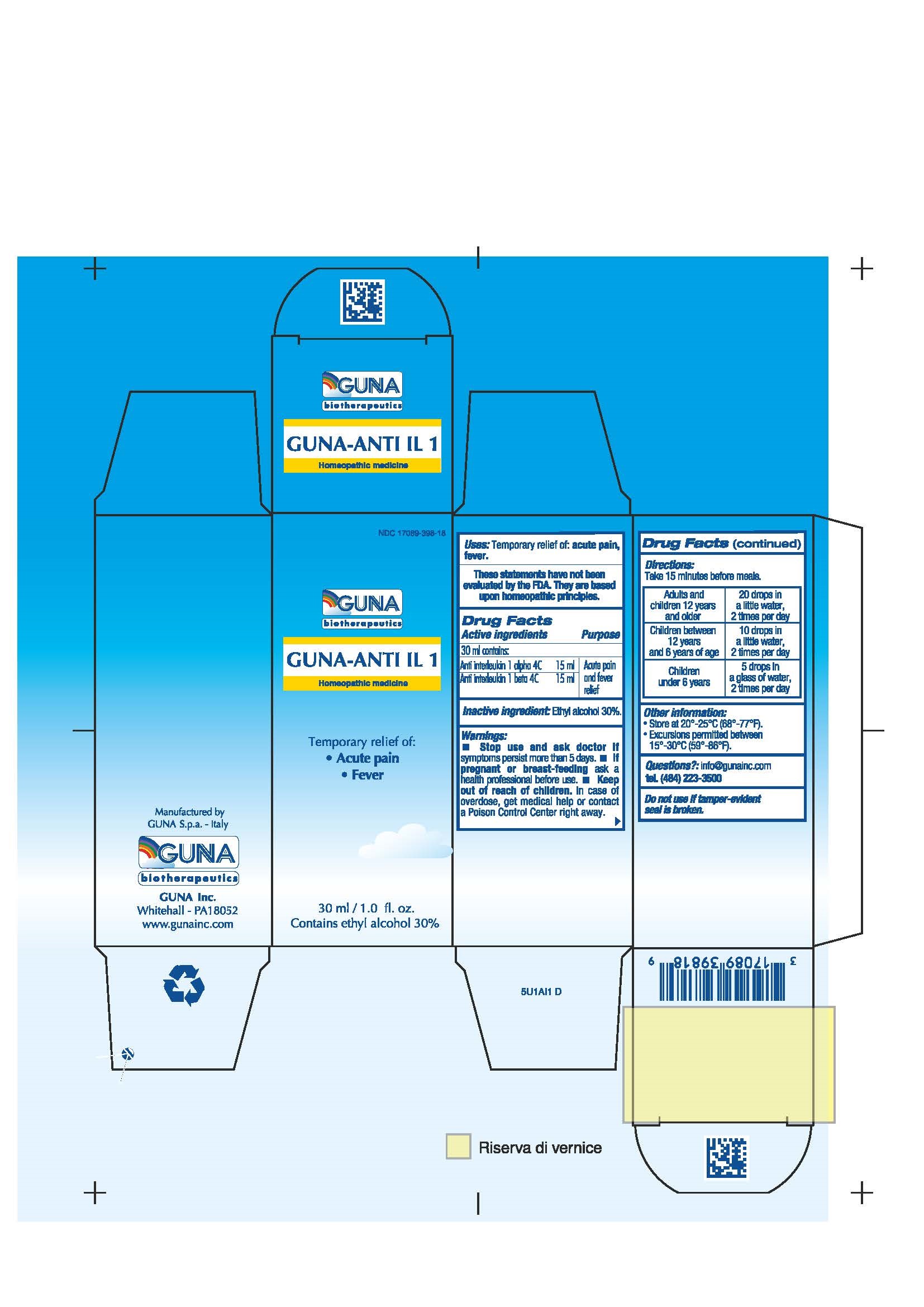
ACTIVE INGREDIENTS/PURPOSE
ANTI INTERLEUKIN 1 ALPHA 4C ACUTE PAIN AND FEVER RELIEF
ANTI INTERLEUKIN 1 BETA 4C ACUTE PAIN AND FEVER RELIEF
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health professional before use.
- Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
- Contains ethyl alcohol 30%
DIRECTIONS
Adults and children 12 years and older 20 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children between 12 years and 6 years of age 10 drops twice a day in a little water. Hold in the mouth for about 30 seconds then swallow.
Children under 6 years 5 drops twice a day in a glass of water.