FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
Apomorphine hydrochloride injection is indicated for the acute, intermittent treatment of hypomobility, "off" episodes ("end-of-dose wearing off" and unpredictable "on/off" episodes) in patients with advanced Parkinson's disease. Apomorphine hydrocloride inejction has been studied as an adjunct to other medications [see Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Apomorphine hydrocloride is indicated for subcutaneous administration only [see Warnings and Precautions (5.1)] and only by a multiple-dose APOKYN® Pen with supplied cartridges. The APOKYN® Pen is supplied separately by a different manufacturer. The initial dose and dose titrations should be performed by a healthcare provider. Blood pressure and pulse should be measured in the supine and standing position before and after dosing.
A caregiver or patient may administer apomorphine hydrocloride if a healthcare provider determines that it is appropriate. Instruct patients to follow the directions provided in the Patients Instructions For Use. Because the APOKYN® Pen has markings in milliliters (mL), the prescribed dose of apomorphine hydrocloride should be expressed in mL to avoid confusion.
Visually inspect the apomorphine hydrocloride drug product through the viewing window for particulate matter and discoloration prior to administration. The solution should not be used if discolored (it should be colorless), or cloudy, or if foreign particles are present. Rotate the injection site and use proper aseptic technique [see How Supplied/Storage and Handling (16) and Patient Counseling Information (17)].
2.2 Premedication and Concomitant Medication
Because of the high incidence of nausea and vomiting with apomorphine hydrocloride treatment, an antiemetic, e.g., trimethobenzamide 300 mg three times a day, should be started 3 days prior to the initial dose of apomorphine hydrocloride [see Warnings and Precautions (5.2)]. Treatment with trimethobenzamide should only be continued as long as necessary to control nausea and vomiting, and generally no longer than two months after initiation of treatment with apomorphine hydrocloride, as trimethobenzamide increases the incidence of somnolence, dizziness and falls in patients treated with apomorphine hydrocloride [see Warnings and Precautions (5.2)].
Based on reports of profound hypotension and loss of consciousness when apomorphine was administered with ondansetron, the concomitant use of apomorphine with drugs of the 5HT3 antagonist class including antiemetics (for example, ondansetron, granisetron, dolasetron, palonosetron) and alosetron are contraindicated [see Contraindications (4)].
2.3 Dosing Information
The recommended starting dose of apomorphine hydrocloride is 0.2 mL (2 mg). Titrate on the basis of effectiveness and tolerance, up to a maximum recommended dose of 0.6 mL (6 mg) [see Clinical Studies (14)].
There is no evidence from controlled trials that doses greater than 0.6 mL (6 mg) gave an increased effect and therefore, individual doses above 0.6 mL (6 mg) are not recommended. The average frequency of dosing in the development program was 3 times per day. There is limited experience with single doses greater than 0.6 mL (6 mg), dosing more than 5 times per day and with total daily doses greater than 2 mL (20 mg).
Begin dosing when patients are in an "off" state. The initial dose should be a 0.2 mL (2 mg) test dose in a setting where medical personnel can closely monitor blood pressure and pulse. Both supine and standing blood pressure and pulse should be checked pre-dose and at 20 minutes, 40 minutes, and 60 minutes post-dose (and after 60 minutes, if there is significant hypotension at 60 minutes). Patients who develop clinically significant orthostatic hypotension in response to this test dose of apomorphine hydrocloride should not be considered candidates for treatment with apomorphine hydrocloride.
If the patient tolerates the 0.2 mL (2 mg) dose, and responds adequately, the starting dose should be 0.2 mL (2 mg), used on an as needed basis to treat recurring "off" episodes. If needed, the dose can be increased in 0.1 mL (1 mg) increments every few days on an outpatient basis.
The general principle guiding subsequent dosing (described in detail below) is to determine that the patient needs and can tolerate a higher test dose, 0.3 mL or 0.4 mL (3 mg or 4 mg, respectively) under close medical supervision. A trial of outpatient dosing may follow (periodically assessing both efficacy and tolerability), using a dose 0.1 mL (1 mg) lower than the tolerated test dose.
If the patient tolerates the 0.2 mL (2 mg) test dose but does not respond adequately, a dose of 0.4 mL (4 mg) may be administered under medical supervision, at least 2 hours after the initial test dose, at the next observed "off" period. If the patient tolerates and responds to a test dose of 0.4 mL (4 mg), the initial maintenance dose should be 0.3 mL (3 mg) used on an as needed basis to treat recurring "off" episodes as an outpatient. If needed, the dose can be increased in 0.1 mL (1 mg) increments every few days on an outpatient basis.
If the patient does not tolerate a test dose of 0.4 mL (4 mg), a test dose of 0.3 mL (3 mg) may be administered during a separate "off" period under medical supervision, at least 2 hours after the previous dose. If the patient tolerates the 0.3 mL (3 mg) test dose, the initial maintenance dose should be 0.2 mL (2 mg) used on an as needed basis to treat existing "off" episodes. If needed, and the 0.2 mL (2 mg) dose is tolerated, the dose can be increased to 0.3 mL (3 mg) after a few days. In such a patient, the dose should ordinarily not be increased to 0.4 mL (4 mg) on an outpatient basis.
2.4 Dosing in Patients with Renal Impairment
For patients with mild and moderate renal impairment, the test dose and starting dose should be reduced to 0.1 mL (1 mg) [see Clinical Pharmacology (12.3) and Use in Specific Populations (8.6)].
2.5 Re-treatment and Interruption in Therapy
If a single dose of apomorphine hydrocloride is ineffective for a particular "off" period, a second dose should not be given for that "off" episode. The efficacy of the safety of administering a second dose for a single "off" episode has not been studied systematically. Do not administer a repeat dose of apomorphine hydrocloride sooner than 2 hours after the last dose.
Patients who have an interruption in therapy of more than a week should be restarted on a 0.2 mL (2 mg) dose and gradually titrated to effect and tolerability.
3 DOSAGE FORMS AND STRENGTHS
Apomorphine hydrocloride 30 mg/3 mL (10 mg/mL) containing apomorphine hydrochloride (as apomorphine hydrochloride hemihydrate), USP is supplied as a clear, colorless, sterile, solution in a 3 mL (30 mg) cartridge. The 3 mL (30 mg) glass cartridge for single-patient-use is used with a manual reusable pen injector (APOKYN® Pen) which is supplied separately by a different manufacturer. A single cartridge, pen and needle can deliver doses up to 1 mL (10 mg) in 0.02 mL (0.2 mg) increments. The APOKYN® pen injector is provided in a package with six needles.
4 CONTRAINDICATIONS
Apomorphine hydrocloride is contraindicated in patients:
- Using concomitant drugs of the 5HT3 antagonist class including antiemetics (e.g., ondansetron, granisetron, dolasetron, palonosetron) and alosetron [see Drug Interactions (7.1)]. There have been reports of profound hypotension and loss of consciousness when apomorphine hydrocloride was administered with ondansetron.
- With hypersensitivity/allergic reaction to apomorphine or to any of the excipients of apomorphine hydrocloride, including a sulfite (i.e., sodium metabisulfite). Angioedema or anaphylaxis may occur [see Warnings and Precautions (5.12)].
5 WARNINGS AND PRECAUTIONS
5.1 Serious Adverse Reactions After Intravenous Administration
Following intravenous administration of apomorphine hydrocloride, serious adverse reactions including thrombus formation and pulmonary embolism due to intravenous crystallization of apomorphine have occurred. Consequently, apomorphine hydrocloride should not be administered intravenously.
5.2 Nausea and Vomiting
Apomorphine hydrocloride causes severe nausea and vomiting when it is administered at recommended doses. Because of this, in domestic clinical studies, 98% of all patients were pre-medicated with trimethobenzamide, an antiemetic, for three days prior to study enrollment, and were then encouraged to continue trimethobenzamide for at least 6 weeks. Even with the use of concomitant trimethobenzamide in clinical studies, 31% and 11% of the apomorphine hydrocloride-treated patients had nausea and vomiting, respectively, and 3% and 2% of the patients discontinued apomorphine hydrocloride due to nausea and vomiting, respectively. Among 522 patients treated, 262 (50%) discontinued trimethobenzamide while continuing apomorphine hydrocloride. The average time to discontinuation of trimethobenzamide was about 2 months (range: 1 day to 33 months). For the 262 patients who discontinued trimethobenzamide, 249 patients continued apomorphine without trimethobenzamide for a duration of follow-up that averaged 1 year (range: 0 years to 3 years).
The effect of trimethobenzamide on reducing nausea and vomiting during treatment with apomorphine hydrocloride was evaluated in a 12-week, placebo-controlled study in 194 patients. The study suggests that trimethobenzamide reduces the incidence of nausea and vomiting during the first 4 weeks of apomorphine hydrocloride treatment (incidence of nausea and vomiting 43% on trimethobenzamide vs. 59% on placebo). However, over the 12-week period, compared with placebo, patients treated with trimethobenzamide had a greater incidence of somnolence (19% for trimethobenzamide vs. 12% for placebo), dizziness (14% for trimethobenzamide vs. 8% for placebo), and falls (8% for trimethobenzamide vs. 1% for placebo). Therefore, the benefit of treatment with trimethobenzamide must be balanced with the risk for those adverse events, and treatment with trimethobenzamide should only be continued as long as necessary to control nausea and vomiting, and generally no longer than two months.
The ability of concomitantly administered antiemetic drugs (other than trimethobenzamide) has not been studied. Antiemetics with anti-dopaminergic actions (e.g., haloperidol, chlorpromazine, promethazine, prochlorperazine, metaclopramide) have the potential to worsen the symptoms in patients with Parkinson's disease and should be avoided.
5.3 Falling Asleep During Activities of Daily Living and Somnolence
There have been reports in the literature of patients treated with apomorphine hydrocloride subcutaneous injections who suddenly fell asleep without prior warning of sleepiness while engaged in activities of daily living. Somnolence is commonly associated with apomorphine hydrocloride, and it is reported that falling asleep while engaged in activities of daily living always occurs in a setting of pre-existing somnolence, even if patients do not give such a history. Somnolence was reported in 35% of patients treated with apomorphine hydrocloride and in none of the patients in the placebo group. Prescribers should reassess patients for drowsiness or sleepiness, especially since some of the events occur well after the start of treatment. Prescribers should also be aware that patients may not acknowledge drowsiness or sleepiness until directly questioned about drowsiness or sleepiness during specific activities.
Before initiating treatment with apomorphine hydrocloride, advise patients of the risk of drowsiness and ask them about factors that could increase the risk with apomorphine hydrocloride, such as concomitant sedating medications and the presence of sleep disorders. If a patient develops significant daytime sleepiness or falls asleep during activities that require active participation (e.g., conversations, eating, etc.), apomorphine hydrocloride should ordinarily be discontinued. If a decision is made to continue apomorphine hydrocloride, patients should be advised not to drive and to avoid other potentially dangerous activities. There is insufficient information to determine whether dose reduction will eliminate episodes of falling asleep while engaged in activities of daily living.
5.4 Syncope/Hypotension/Orthostatic Hypotension
In clinical studies, approximately 2% of apomorphine hydrochloride-treated patients experienced syncope. Dopamine agonists, including apomorphine hydrochloride, may cause orthostatic hypotension at any time but especially during dose escalation. Patients with Parkinson's disease may also have an impaired capacity to respond to an orthostatic challenge. For these reasons, Parkinson's disease patients being treated with dopaminergic agonists ordinarily require careful monitoring for signs and symptoms of orthostatic hypotension, especially during dose escalation, and should be informed of this risk.
Patients undergoing titration of apomorphine hydrochloride showed an increased incidence (from 4% pre-dose to 18% post-dose) of systolic orthostatic hypotension (≥ 20 mmHg decrease) when evaluated at various times after in-office dosing. A small number of patients developed severe systolic orthostatic hypotension (≥ 30 mm Hg decrease and systolic BP ≤ 90 mmHg) after subcutaneous apomorphine injection. In clinical trials of apomorphine hydrochloride in patients with advanced Parkinson's disease, 59 of 550 patients (11%) had orthostatic hypotension, hypotension, and/or syncope. These events were considered serious in 4 patients (< 1%) and resulted in withdrawal of apomorphine hydrochloride in 10 patients (2%). These events occurred both with initial dosing and during long-term treatment. Whether or not hypotension contributed to other significant adverse events seen (e.g., falls), is unknown. Apomorphine hydrochloride causes dose-related decreases in systolic (SBP) and diastolic blood pressure (DBP) [see Clinical Pharmacology (12.2)].
In a study of healthy subjects, the hypotensive effect of apomorphine hydrochloride on systolic and diastolic blood pressure) was exacerbated by the concomitant use of alcohol or sublingual nitroglycerin (0.4 mg). Patients should avoid alcohol when using apomorphine hydrochloride [see Drug Interactions (7.3)]. Patients taking apomorphine hydrochloride should lie down before and after taking sublingual nitroglycerin. Other vasodilators and antihypertensives may also increase the hypotensive effects of apomorphine hydrochloride. Monitor blood pressure for hypotension and orthostatic hypotension in patients taking apomorphine hydrochloride with concomitant antihypertensive medications or vasodilators [see Drug Interactions (7.2, 7.3)].
5.5 Falls
Patients with Parkinson's disease (PD) are at risk of falling due to underlying postural instability, possible autonomic instability, and syncope caused by the blood pressure lowering effects of the drugs used to treat PD. Subcutaneous apomorphine hydrocloride might increase the risk of falling by simultaneously lowering blood pressure and altering mobility [see Clinical Pharmacology (12.2)].
In clinical trials, 30% of patients had events that could reasonably be considered falls and about 5% of patients had falls that were considered serious.
5.6 Hallucinations / Psychotic-Like Behavior
In clinical studies, hallucinations were reported by 14% of the apomorphine hydrocloride-treated patients. In one randomized, double-blind, placebo-controlled study, hallucinations or confusion occurred in 10% of patients treated with apomorphine hydrocloride and 0% of patients treated with placebo. Hallucinations resulted in discontinuation of apomorphine hydrocloride in 1% of patients.
Postmarketing reports indicate that patients may experience new or worsening mental status and behavioral changes, which may be severe, including psychotic-like behavior after starting or increasing the dose of apomorphine hydrocloride. Other drugs prescribed to improve the symptoms of Parkinson's disease can have similar effects on thinking and behavior. This abnormal thinking and behavior can consist of one or more of a variety of manifestations, including paranoid ideation, delusions, hallucinations, confusion, disorientation, aggressive behavior, agitation, and delirium.
Patients with a major psychotic disorder should ordinarily not be treated with apomorphine hydrocloride because of the risk of exacerbating psychosis. In addition, certain medications used to treat psychosis may exacerbate the symptoms of Parkinson's disease and may decrease the effectiveness of apomorphine hydrocloride [see Drug Interactions (7.3)].
5.7 Dyskinesias
Apomorphine hydrocloride may cause dyskinesia or exacerbate pre-existing dyskinesia. In clinical studies, dyskinesia or worsening of dyskinesia was reported in 24% of patients. Overall, 2% of apomorphine hydrocloride-treated patients withdrew from studies due to dyskinesias.
5.8 Impulse Control/Compulsive Behaviors
Case reports suggest that patients can experience intense urges to gamble, increased sexual urges, intense urges to spend money uncontrollably, and other intense urges and the inability to control these urges while taking one or more of the medications, including apomorphine hydrocloride, that increase central dopaminergic tone and that are generally used for the treatment of Parkinson's disease. In some cases, although not all, these urges were reported to have stopped when the dose was reduced or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending or other urges while being treated with apomorphine hydrocloride. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking apomorphine hydrocloride.
5.9 Coronary Events
In clinical studies, 4% of patients treated with apomorphine hydrocloride experienced angina, myocardial infarction, cardiac arrest and/or sudden death; some cases of angina and myocardial infarction occurred in close proximity to apomorphine hydrocloride dosing (within 2 hours), while other cases of cardiac arrest and sudden death were observed at times unrelated to dosing. apomorphine hydrocloride has been shown to reduce resting systolic and diastolic blood pressure and may have the potential to exacerbate coronary (and cerebral) ischemia in patients with known cardiovascular and cerebrovascular disease. If patients develop signs and symptoms of coronary or cerebral ischemia, prescribers should re-evaluate the continued use of apomorphine hydrocloride.
5.10 QTc Prolongation and Potential for Proarrhythymic Effects
There is a dose related prolongation of QTc interval after apomorphine exposure similar to that achieved with therapeutic doses of apomorphine hydrocloride [see Clinical Pharmacology (12.2)]. Doses greater than 6 mg do not provide additional clinical benefit and are not recommended.
Drugs that prolong the QTc interval have been associated with torsades de pointes and sudden death. The relationship of QTc prolongation to torsades de pointes is clearest for larger increases (20 msec and greater), but it is possible that smaller QTc prolongations may also increase risk, or increase it in susceptible individuals, such as those with hypokalemia, hypomagnesemia, bradycardia, concomitant use of other drugs that prolong the QTc interval, or genetic predisposition (e.g., congenital prolongation of the QT interval). Although torsades de pointes has not been observed in association with the use of apomorphine hydrocloride at recommended doses in clinical studies, experience is too limited to rule out an increased risk. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes.
The risks and benefits of apomorphine hydrocloride treatment should be considered prior to initiating treatment with apomorphine hydrocloride in patients with risk factors for prolonged QTc.
5.11 Withdrawal-Emergent Hyperpyrexia and Confusion
A symptom complex resembling neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in antiparkinsonian therapy.
5.12 Hypersensitivity
Hypersensitivity/allergic reactions characterized by urticaria, rash, pruritus, and/or various manifestations of angioedema may occur because of apomorphine hydrocloride or because of its sulfite excipient. apomorphine hydrocloride contains sodium metabisulfite, a sulfite that may cause allergic-type reactions, including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
5.13 Fibrotic Complications
Cases of retroperitoneal fibrosis, pulmonary infiltrates, pleural effusion, pleural thickening, and cardiac valvulopathy have been reported in some patients treated with ergot-derived dopaminergic agents. While these complications may resolve when the drug is discontinued, complete resolution does not always occur. Although these adverse reactions are believed to be related to the ergoline structure of these dopamine agonists, whether other, nonergot derived dopamine agonists, such as apomorphine hydrocloride, can cause these reactions is unknown.
5.14 Priapism
apomorphine hydrocloride may cause prolonged painful erections in some patients. In clinical studies, painful erections were reported by 3 of 361 apomorphine hydrocloride-treated men, and one patient withdrew from apomorphine hydrocloride therapy because of priapism. Although no patients in the clinical studies required surgical intervention, severe priapism may require surgical intervention.
5.15 Retinal Pathology in Albino Rats
In a 2-year carcinogenicity study of apomorphine in albino rat, retinal atrophy was detected at all subcutaneous doses tested (up to 0.8 mg/kg/day or 2 mg/kg/day in males or females, respectively; less than the maximum recommended human dose of 20 mg/day on a body surface area [mg/m2] basis). Retinal atrophy/degeneration has been observed in albino rats treated with other dopamine agonists for prolonged periods (generally during 2-year carcinogenicity studies). Retinal findings were not observed in a 39-week subcutaneous toxicity study of apomorphine in monkey at doses up to 1.5 mg/kg/day, a dose similar to the MRHD on a mg/m2 basis. The clinical significance of the finding in rat has not been established but cannot be disregarded because disruption of a mechanism that is universally present in vertebrates (e.g., disk shedding) may be involved.
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in more detail in the Warnings and Precautions section of labeling:
- Serious Adverse Reactions After Intravenous Administration [see Warnings and Precautions (5.1)]
- Nausea and Vomiting [see Warnings and Precautions (5.2)]
- Falling Asleep During Activities of Daily Living and Somnolence [see Warnings and Precautions (5.3)]
- Syncope/Hypotension/Orthostatic Hypotension [see Warnings and Precautions (5.4)]
- Falls [see Warnings and Precautions (5.5)]
- Hallucinations/Psychotic-Like Behavior [see Warnings and Precautions (5.6)]
- Dyskinesias [see Warnings and Precautions (5.7)]
- Impulse Control/Compulsive Behaviors [see Warnings and Precautions (5.8)]
- Coronary Events [see Warnings and Precautions (5.9)]
- QTc Prolongation and Potential for Proarrhythymic Effects [see Warnings and Precautions (5.10)]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see Warnings and Precautions (5.11)]
- Hypersensitivity [see Warnings and Precautions (5.12)]
- Fibrotic Complications [see Warnings and Precautions (5.13)]
- Priapism [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the incidence of adverse reactions (number of unique patients experiencing an adverse reaction associated with treatment per total number of patients treated) observed in the clinical trials of a drug cannot be directly compared to the incidence of adverse reactions in the clinical trials of another drug and may not reflect the incidence of adverse reactions observed in practice.
In placebo-controlled trials, most patients received only one subcutaneous dose of apomorphine hydrocloride. All patients received concomitant levodopa and 86% received a concomitant dopamine agonist. All patients had some degree of spontaneously occurring periods of hypomobility ("off episodes") at baseline.
The most common adverse reactions (apomorphine hydrocloride incidence at least 10% greater than placebo incidence) observed in a placebo-controlled trial were yawning, drowsiness/somnolence, dyskinesias, dizziness/postural hypotension, rhinorrhea, nausea and/or vomiting, hallucination/confusion, and edema/swelling of extremities.
Table 1 presents the most common adverse reactions reported by apomorphine hydrocloride-naïve Parkinson's disease patients who were enrolled in a randomized placebo-controlled, parallel group trial and who were treated for up to 4 weeks (Study 1) [see Clinical Studies (14)]. Individual apomorphine hydrocloride doses in this trial ranged from 2 mg to 10 mg, and were titrated to achieve tolerability and control of symptoms.
| Apomorphine Hydrocloride (n = 20) | PLACEBO (n = 9) | |
|---|---|---|
| % | % | |
| Yawning | 40 | 0 |
| Dyskinesias | 35 | 11 |
| Drowsiness or Somnolence | 35 | 0 |
| Nausea and/or Vomiting | 30 | 11 |
| Dizziness or Postural Hypotension | 20 | 0 |
| Rhinorrhea | 20 | 0 |
| Chest Pain/Pressure/Angina | 15 | 11 |
| Hallucination or Confusion | 10 | 0 |
| Edema/Swelling of Extremities | 10 | 0 |
Other Adverse Reactions
Injection Site Reactions
Patients treated with apomorphine hydrocloride subcutaneous injections during clinical studies, 26% of patients had injection site reactions, including bruising (16%), granuloma (4%), and pruritus (2%).
In addition to those in Table 1, the most common adverse reactions in pooled apomorphine hydrocloride trials (occurring in at least 5% of the patients) in descending order were injection site reaction, fall, arthralgia, insomnia, headache, depression, urinary tract infection, anxiety, congestive heart failure, limb pain, back pain, Parkinson's disease aggravated, pneumonia, confusion, sweating increased, dyspnea, fatigue, ecchymosis, constipation, diarrhea, weakness, and dehydration.
7 DRUG INTERACTIONS
7.1 5HT3 Antagonists
Based on reports of profound hypotension and loss of consciousness when apomorphine hydrocloride was administered with ondansetron, the concomitant use of apomorphine hydrocloride with 5HT3 antagonists including antiemetics (for example, ondansetron, granisetron, dolasetron, palonosetron) and alosetron, is contraindicated.
7.2 Antihypertensive Medications and Vasodilators
In clinical studies, the following adverse events were experienced more commonly in patients receiving concomitant antihypertensive medications or vasodilators (n=94) than in patients not receiving these medications (n=456): hypotension (10% vs 4%)[see Warnings and Precautions (5.4)], myocardial infarction (3% vs 1%), serious pneumonia (5% vs 3%), serious falls (9% vs 3%), and bone and joint injuries (6% vs 2%). Some of the events may be related to the increased incidence of hypotension in patients receiving concomitant antihypertensive medications or vasodilators [see Warnings and Precautions (5.4, 5.5)].
Concomitant administration of 0.4 mg sublingual nitroglycerin with apomorphine hydrocloride in healthy subjects causes greater decreases in blood pressure compared to apomorphine hydrocloride alone. When nitroglycerin and apomorphine hydrocloride were concomitantly administered to healthy subjects, the mean largest decrease (the mean of each subject's largest drop in blood pressure measured within the 6-hour period following administration of apomorphine hydrocloride) in supine systolic and diastolic blood pressure (measured over 6 hours) was 9.7 mm Hg and 9.3 mm Hg, respectively [see Clinical Pharmacology (12.3)]. The mean largest decrease in standing systolic and diastolic blood pressure was 14.3 mm Hg and 13.5 mm Hg, respectively. Some individuals experienced very large decreases in standing systolic and diastolic blood pressure, up to a maximum decrease of 65 mm Hg and 43 mm Hg, respectively.
In comparison, the mean largest decrease in supine systolic and diastolic blood pressure when apomorphine hydrocloride was administered alone was 6.1 mm Hg and 7.3 mm Hg, respectively, and in standing systolic and diastolic blood pressure was 6.7 mm Hg and 8.4 mm Hg, respectively.
Patients taking apomorphine hydrocloride should lie down before and after taking sublingual nitroglycerin [see Warnings and Precautions (5.4)].
7.3 Alcohol
Concomitant administration of high dose (0.6 g/kg) or low dose (0.3 g/kg) ethanol with apomorphine hydrocloride in healthy subjects causes greater decreases in blood pressure compared to apomorphine hydrocloride alone.
When high dose ethanol and apomorphine hydrocloride were concomitantly administered to healthy subjects, the mean largest decrease (the mean of each subject's largest drop in blood pressure measured within the 6-hour period following administration of apomorphine hydrocloride) for supine systolic and diastolic blood pressure was 9.1 mm Hg and 10.5 mm Hg, respectively [see Clinical Pharmacology (12.3)]. The mean largest standing systolic and diastolic blood pressure decrease was 11.3 mm Hg and 12.6 mm Hg, respectively. In some individuals, the decrease was as high as 61 mm Hg and 51 mm Hg, respectively, for standing systolic and diastolic blood pressure.
When low dose ethanol and apomorphine hydrocloride were concomitantly administered, the mean largest decrease in supine systolic and diastolic blood pressure was 10.2 mm Hg and 9.9 mm Hg, respectively. The mean largest decrease in standing systolic and diastolic blood pressure was 8.4 mm Hg and 7.1 mm Hg, respectively.
In comparison, the mean largest decrease in supine systolic and diastolic blood pressure when apomorphine hydrocloride was administered alone was 6.1 mm Hg and 7.3 mm Hg, respectively, and in standing systolic and diastolic blood pressure was 6.7 mm Hg 8.4 mm Hg, respectively.
Patients should avoid drinking alcohol after using apomorphine hydrocloride [see Warnings and Precautions (5.4)].
7.4 Dopamine Antagonists
Since apomorphine hydrocloride is a dopamine agonist, it is possible that concomitant use of dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of apomorphine hydrocloride. Patients with major psychotic disorders, treated with neuroleptics, should be treated with dopamine agonists only if the potential benefits outweigh the risks.
7.5 Drugs Prolonging the QT/QTc Interval
Caution should be exercised when prescribing apomorphine hydrocloride concomitantly with drugs that prolong the QT/QTc interval [see Warnings and Precautions (5.10)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with use of apomorphine hydrocloride in pregnant women. In animal reproduction studies, apomorphine had adverse developmental effects in rats (increased neonatal deaths) and rabbits (increased incidence of malformation) when administered during pregnancy at clinically relevant doses. These doses were also associated with maternal toxicity [see Data]. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
No adverse developmental effects were observed when apomorphine (0.3, 1, or 3 mg/kg/day) was administered by subcutaneous injection to pregnant rats throughout organogenesis; the highest dose tested is 1.5 times the maximum recommended human dose (MRHD) of 20 mg/day on a mg/m2 basis. Administration of apomorphine (0.3, 1, or 3 mg/kg/day) by subcutaneous injection to pregnant rabbits throughout organogenesis resulted in an increased incidence of malformations of the heart and/or great vessels at the mid and high doses; maternal toxicity was observed at the highest dose tested. The no-effect dose for adverse developmental effects is less than the MRHD on a mg/m2 basis.
Apomorphine (0.3, 1, or 3 mg/kg/day), administered by subcutaneous injection to females throughout gestation and lactation, resulted in increased offspring mortality at the highest dose tested, which was associated with maternal toxicity. There were no effects on developmental parameters or reproductive performance in surviving offspring. The no-effect dose for developmental toxicity (1 mg/kg/day) is less than the MRHD on a mg/m2 basis.
8.2 Lactation
Risk Summary
There are no data on the presence of apomorphine in human milk, the effects of apomorphine on the breastfed infant, or the effects of apomorphine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for apomorphine hydrocloride and any potential adverse effects on the breastfed infant from apomorphine hydrocloride or from the underlying maternal condition.
8.5 Geriatric Use
In the apomorphine hydrocloride clinical development program, there were 239 patients less than age 65 treated with apomorphine hydrocloride and 311 patients who were age 65 or older. Confusion and hallucinations were reported more frequently with patients age 65 and older compared to patients with less than age 65. Serious adverse reactions (life-threatening events or events resulting in hospitalization and/or increased disability) were also more common in patients age 65 and older. Patients age 65 and older were more likely to fall (experiencing bone and joint injuries), have cardiovascular events, develop respiratory disorders, and have gastrointestinal events. Patients age 65 and above were also more likely to discontinue apomorphine hydrocloride treatment as a result of one or more adverse reactions.
8.6 Renal Impairment
The starting apomorphine hydrocloride dose should be reduced in patients with mild or moderate renal impairment because the concentration and exposure (Cmax and AUC) are increased in these patients. Studies in subjects with severe renal impairment have not been conducted [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Caution should be exercised when administrating apomorphine hydrocloride to patients with mild and moderate hepatic impairment because of the increased Cmax and AUC in these patients. Closely monitor patients with mild and moderate hepatic impairment. Studies of subjects with severe hepatic impairment have not been conducted [see Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
In premarketing clinical experience, apomorphine hydrocloride did not reveal any tendency for a withdrawal syndrome or any drug-seeking behavior. However, there are rare postmarketing reports of abuse of medications containing apomorphine hydrocloride or levodopa. In general, these reports consist of patients taking increasing doses of medication in order to achieve a euphoric state.
10 OVERDOSAGE
A 62-year-old man accidentally injected 25 mg of apomorphine hydrocloride subcutaneously. After 3 minutes, the patient felt nauseated and lost consciousness for 20 minutes. Afterwards, he was alert with a heart rate 40/minute and a supine blood pressure of 90/50. He recovered completely within an hour.
11 DESCRIPTION
apomorphine hydrocloride (apomorphine hydrochloride injection) contains apomorphine hydrochloride, a non-ergoline dopamine agonist. Apomorphine hydrochloride is chemically designated as 6aβ-Aporphine-10,11-diol hydrochloride hemihydrate with a molecular formula of C17H17NO2 ∙ HCl ∙ ½ H2O. Its structural formula and molecular weight are:
Figure 1: Structural Formula and Molecular Weight of Apomorphine
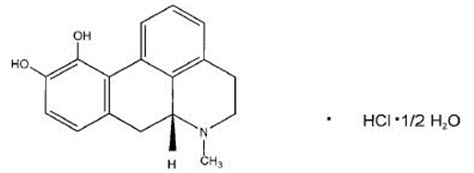
M.W. 312.79
Apomorphine hydrochloride appears as minute, white or grayish-white glistening crystals or as white powder that is soluble in water at 80°C.
Apomorphine hydrocloride is a clear, colorless, sterile solution for subcutaneous injection and is available in 3 mL (30 mg) multi-dose cartridges. Each mL of solution contains 10 mg of apomorphine hydrochloride, USP as apomorphine hydrochloride hemihydrate (equivalent to 8.55 mg apomorphine), 1 mg of sodium metabisulfite, NF and 5 mg of benzyl alcohol, NF (preservative) in water for injection, USP. In addition, each mL of solution may contain sodium hydroxide, NF and/or hydrochloric acid, NF to adjust the pH of the solution.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Apomorphine hydrocloride is a non-ergoline dopamine agonist with high in vitro binding affinity for the dopamine D4 receptor, and moderate affinity for the dopamine D2, D3, and D5, and adrenergic α1D, α2B, α2C receptors. The precise mechanism of action of apomorphine hydrocloride as a treatment for Parkinson's disease is unknown, although it is believed to be due to stimulation of post-synaptic dopamine D2-type receptors within the caudate-putamen in the brain.
12.2 Pharmacodynamics
Prolongation of the QTc Interval
In a thorough QT study at exposures similar to those achieved with the recommended dosing, apomorphine resulted in a prolongation of QTcF of 10 msec (90% upper confidence interval of 16 msec). The thorough QT study also identified a significant exposure-response relationship between apomorphine concentration and QTcF.
Decreases in Blood Pressure
Dose-dependent mean decreases in systolic blood pressure ranged from 5 mm Hg to 16 mm Hg after administration of apomorphine hydrocloride 2 mg and 10 mg, respectively. Dose-dependent mean decreases in diastolic blood pressure ranged from 3 mm Hg to 8 mm Hg after administration of apomorphine hydrocloride 2 mg and 10 mg, respectively. These changes were observed 20 minutes after dosing, and were maximal between 20 and 40 minutes after dosing. Lesser, but still noteworthy blood pressure decreases persisted up to at least 90 minutes after dosing. Effects on blood pressure are additive when apomorphine hydrocloride is coadministered with nitroglycerin or alcohol [see Drug Interactions (7.3, 7.4)].
12.3 Pharmacokinetics
Absorption
Apomorphine hydrochloride is a lipophilic compound that is rapidly absorbed (time to peak concentration ranges from 10 minutes to 60 minutes) following subcutaneous administration into the abdominal wall. After subcutaneous administration, apomorphine appears to have bioavailability equal to that of an intravenous administration. Apomorphine exhibits linear pharmacokinetics over a dose range of 2 mg to 8 mg following a single subcutaneous injection of apomorphine hydrocloride into the abdominal wall in patients with idiopathic Parkinson's disease.
Distribution
The plasma-to-whole blood apomorphine concentration ratio is equal to one. Mean (range) apparent volume of distribution was 218 L (123 L to 404 L). Maximum concentrations in cerebrospinal fluid (CSF) are less than 10% of maximum plasma concentrations and occur 10 minutes to 20 minutes later.
Metabolism and Elimination
The mean apparent clearance (range) is 223 L/hr (125 L/hr to 401 L/hr) and the mean terminal elimination half-life is about 40 minutes (range about 30 minutes to 60 minutes).
The route of metabolism in humans is not known. Potential routes of metabolism in humans include sulfation, N-demethylation, glucuronidation and oxidation. In vitro, apomorphine undergoes rapid autooxidation.
Specific Populations
The clearance of apomorphine does not appear to be influenced by age, gender, weight, duration of Parkinson's disease, levodopa dose, or duration of therapy.
Renal Impairment
In a study comparing renally-impaired subjects (moderately impaired as determined by estimated creatinine clearance) to healthy matched volunteers, the AUC0-∞ and Cmax values were increased by approximately 16% and 50%, respectively, following a single subcutaneous administration of apomorphine hydrocloride into the abdominal wall. The mean time to peak concentrations and the mean terminal half-life of apomorphine were unaffected by the renal status of the individual. Studies in subjects with severe renal impairment have not been conducted. The starting dose for patients with mild or moderate renal impairment should be reduced [see Dosage and Administration (2.4) and Use in Specific Populations (8.6)].
Hepatic Impairment
In a study comparing subjects with hepatic impairment (moderately impaired as determined by the Child-Pugh classification method) to healthy matched volunteers, the AUC0-∞ and Cmax values were increased by approximately 10% and 25%, respectively, following a single subcutaneous administration of apomorphine hydrocloride into the abdominal wall. Studies in subjects with severe hepatic impairment have not been conducted [see Dosage and Administration (2.5) and Use in Specific Populations (8.7)].
Drug Interaction Studies
Carbidopa/levodopa
Levodopa pharmacokinetics were unchanged when subcutaneous apomorphine hydrocloride and levodopa were co-administrated in patients. However, motor response differences were significant. The threshold levodopa concentration necessary for an improved motor response was reduced significantly, leading to an increased duration of effect without a change in the maximal response to levodopa therapy.
Ethanol and Nitroglycerin
Co-administration of low dose ethanol (0.3 g/kg) or nitroglycerin (0.4 mg) with apomorphine hydrocloride in healthy subjects did not have a significant impact on the pharmacokinetics of apomorphine, but high dose ethanol (0.6 g/kg), equivalent to approximately 3 standardized alcohol-containing beverages, increased the Cmax of apomorphine by about 63%. However, the hypotensive effect of apomorphine hydrocloride was increased by the concomitant use of alcohol or of sublingual nitroglycerin [see Warnings and Precautions (5.4) and Drug Interactions (7.2, 7.3)].
Other Drugs Eliminated Via Hepatic Metabolism
Based upon an in vitro study, cytochrome P450 enzymes play a minor role in the metabolism of apomorphine. In vitro studies have also demonstrated that drug interactions are unlikely due to apomorphine acting as a substrate, an inhibitor, or an inducer of cytochrome P450 enzymes.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies of apomorphine were conducted in male (0.1, 0.3, or 0.8 mg/kg/day) and female (0.3, 0.8, or 2 mg/kg/day) rats. Apomorphine was administered by subcutaneous injection for 22 months or 23 months, respectively. In males, there was an increase in Leydig cell tumors at the highest dose tested, which is less than the MRHD (20 mg) on a mg/m2 basis. This finding is of questionable significance because the endocrine mechanisms believed to be involved in the production of Leydig cell tumors in rats are not relevant to humans. No drug-related tumors were observed in females; the highest dose tested is similar to the MRHD on a mg/m2 basis.
In a 26-week carcinogenicity study in P53-knockout transgenic mice, there was no evidence of carcinogenic potential when apomorphine was administered by subcutaneous injection at doses up to 20 mg/kg/day (male) or 40 mg/kg/day (female).
Mutagenesis
Apomorphine was mutagenic in the in vitro bacterial reverse mutation (Ames) and the in vitro mouse lymphoma tk assays. Apomorphine was clastogenic in the in vitro chromosomal aberration assay in human lymphocytes and in the in vitro mouse lymphoma tk assay. Apomorphine was negative in the in vivo micronucleus assay in mice.
Impairment of Fertility
Apomorphine was administered subcutaneously at doses up to 3 mg/kg/day (approximately 1.5 times the MRHD on a mg/m2 basis) to male and female rats prior to and throughout the mating period and continuing in females through gestation day 6. There was no evidence of adverse effects on fertility or on early fetal viability. A significant decrease in testis weight was observed in a 39-week study in cynomolgus monkey at all subcutaneous doses tested (0.3, 1, or 1.5 mg/kg/day); the lowest dose tested is less than the MRHD on a mg/m2 basis.
In a published fertility study, apomorphine was administered to male rats at subcutaneous doses of 0.2, 0.8, or 2 mg/kg prior to and throughout the mating period. Fertility was reduced at the highest dose tested.
14 CLINICAL STUDIES
The effectiveness of apomorphine hydrocloride in the acute symptomatic treatment of the recurring episodes of hypomobility, "off" episodes ("end-of-dose wearing off" and unpredictable "on/off" episodes), in patients with advanced Parkinson's disease was established in three randomized, controlled trials of apomorphine hydrocloride given subcutaneously (Studies 1, 2, and 3). At baseline in these trials, the mean duration of Parkinson's disease was approximately 11 years. Whereas all patients were using concomitant L-dopa at baseline, 86% of patients were using a concomitant oral dopaminergic agonist, 31% were using a concomitant catechol-ortho-methyl transferase (COMT) inhibitor, and 10% were using a concomitant monoamine B oxidase inhibitor. Study 1 was conducted in patients who did not have prior exposure to apomorphine hydrocloride (i.e., apomorphine hydrocloride naïve) and Studies 2 and 3 were conducted in patients with at least 3 months of apomorphine hydrocloride use immediately prior to study enrollment. Almost all patients without prior exposure to apomorphine hydrocloride began taking an antiemetic (trimethobenzamide) three days prior to starting apomorphine hydrocloride and 50% of patients were able to discontinue the concomitant antiemetic, on average 2 months after initiating apomorphine hydrocloride.
The change from baseline in Part III (Motor Examination) of the Unified Parkinson's Disease Rating Scale (UPDRS) served as the primary outcome assessment measure in each study. Part III of the UPDRS contains 14 items designed to assess the severity of the cardinal motor findings (e.g., tremor, rigidity, bradykinesia, postural instability, etc.) in patients with Parkinson's disease.
Study 1
Study 1 was a randomized, double-blind, placebo-controlled, parallel-group trial in 29 patients with advanced Parkinson's disease who had at least 2 hours of "off" time per day despite an optimized oral regimen for Parkinson's disease including levodopa and an oral dopaminergic agonist. Patients with atypical Parkinson's disease, psychosis, dementia, hypotension, or those taking dopamine antagonists were excluded from participation. In an office setting, hypomobility was allowed to occur by withholding the patients' Parkinson's disease medications overnight. The following morning, patients (in a hypomobile state) were started on study treatment in a 2:1 ratio (2 mg of apomorphine hydrocloride or placebo given subcutaneously). At least 2 hours after the first dose, patients were given additional doses of study medication until they achieved a "therapeutic response" (defined as a response similar to the patient's response to their usual dose of levodopa) or until 10 mg of apomorphine hydrocloride or placebo equivalent was given. At each injection re-dosing, the study drug dose was increased in 2 mg increments up to 4 mg, 6 mg, 8 mg, 10 mg of apomorphine hydrocloride) or placebo equivalent.
Of the 20 patients randomized to apomorphine hydrocloride, 18 achieved a "therapeutic response" at about 20 minutes. The mean apomorphine hydrocloride dose was 5.4 mg (3 patients on 2 mg, 7 patients on 4 mg, 5 patients on 6 mg, 3 patients on 8 mg, and 2 patients on 10 mg). In contrast, of the 9 placebo-treated patients, none reached a "therapeutic response." The mean change from baseline for UPDRS Part III score for apomorphine hydrocloride group (highest dose) was statistically significant compared to that for the placebo group (Table 2).
| Treatment | Baseline UPDRS Motor Score | Mean Change from Baseline | Difference from placebo |
|---|---|---|---|
| Placebo | 36.3 | - 0.1 | NA |
| Apomorphine Hydrocloride | 39.7 | - 23.9 | - 23.8 |
Study 2
Study 2 used a randomized, placebo-controlled crossover design of 17 patients with Parkinson's disease who had been using apomorphine hydrocloride for at least 3 months. Patients received their usual morning doses of Parkinson's disease medications and were followed until hypomobility occurred, at which time they received either a single dose of subcutaneous apomorphine hydrocloride (at their usual dose) and placebo on different days in random order. UPDRS Part III scores were evaluated over time. The mean dose of apomorphine hydrocloride was 4 mg (2 patients on 2 mg, 9 patients on 3 mg, 2 patients on 4 mg, and 1 patient each on 4.5 mg, 5 mg, 8 mg, and 10 mg). The mean change from baseline UPDRS Part III score for the apomorphine hydrocloride group was statistically significant compared to that for the placebo group (Table 3).
| Treatment | Baseline UPDRS Motor Score | Mean Change from Baseline | Difference from placebo |
|---|---|---|---|
| Placebo | 40.1 | - 3.0 | NA |
| Apomorphine Hydrocloride | 41.3 | - 20.0 | - 17.0 |
Study 3
Study 3 used a randomized withdrawal design in 4 parallel groups from 62 patients (apomorphine hydrocloride-35; Placebo-27) with Parkinson's disease who had been using apomorphine hydrocloride for at least 3 months. Patients were randomized to one of the following 4 treatments dosed once by subcutaneous administration: apomorphine hydrocloride at the usual dose (mean dose 4.6 mg), placebo at a volume matching the usual apomorphine hydrocloride dose, apomorphine hydrocloride at the usual dose + 2 mg (0.2 mL) (mean dose 5.8 mg), or placebo at a volume matching the usual apomorphine hydrocloride dose + 0.2 mL. Patients received their usual morning doses of Parkinson's disease medications and were followed until hypomobility occurred, at which time they received the randomized treatment. apomorphine hydrocloride doses ranged between 2 mg – 10 mg. The mean change from baseline for the apomorphine hydrocloride group for UPDRS Part III scores at 20 minutes post dosing was statistically significant compared to that for the placebo group (Table 4). Figure 2 describes the mean change from baseline in UPDRS Motor Scores over time for pooled apomorphine hydrocloride and placebo administration.
| Treatment | Baseline UPDRS Motor Score | Mean Change from Baseline | Difference from placebo |
|---|---|---|---|
| Placebo (Pooled) | 40.6 | - 7.4 | NA |
| apomorphine hydrocloride (Pooled) | 42.0 | - 24.2 | - 16.8 |
| Figure 2: Mean Change from Baseline in UPDRS Motor Scores of Pooled apomorphine hydrocloride Groups and Placebo Group in Study 3 |
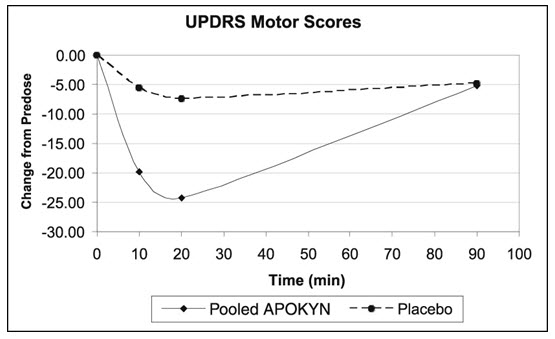 |
In Study 3, the mean changes from baseline for UPDRS Part III scores at 20 minutes post dosing for the apomorphine hydrocloride and higher dose apomorphine hydrocloride groups were 24 and 25, respectively. This result suggests that patients chronically treated at a dose of 4 mg might derive little additional benefit from a dose increment of 2 mg. There was also an increased incidence of adverse reactions in patients randomized to higher apomorphine hydrocloride dose.
16 HOW SUPPLIED/STORAGE AND HANDLING
Apomorphine hydrocloride injection is supplied as a clear, colorless, sterile solution in cartridges, 30 mg/3 mL (10 mg/mL), for single-patient-use with a pen injector (APOKYN® Pen). The APOKYN® Pen is supplied separately by a different manufacturer.
NDC 52817-720-05
Cartons of five 3 mL cartridges
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use)
Administration with the APOKYN® Pen
- Instruct patients and caregivers that the APOKYN® Pen is dosed in milliliters, not milligrams.
- Instruct patients and caregivers that do not already have an APOKYN® Pen, that an APOKYN® Pen is obtained separately from a different manufacturer.
Inform patients and caregivers that it is possible to dial in their usual dose of apomorphine hydrocloride even though the cartridge may contain less than that amount of drug. In this case, they will receive only a partial dose with the injection, and the amount left to inject will appear in the dosing window. To complete the correct dose, patients/caregivers will need to "re-arm" the device and dial in the correct amount of the remaining dose. Patients and caregivers should be alerted to the fact that there may be insufficient drug left in the cartridge to deliver a complete dose (for example, patients and caregivers should be urged to keep records of how many doses they have delivered for each cartridge, so that they can replace any cartridge that has an inadequate amount of drug remaining).
Instruct patients to rotate the injection site and to observe proper aseptic technique.
Advise patients that apomorphine hydrocloride is intended only for subcutaneous injection and must not be given intravenously because of the risk of serious complications such as thrombus formation and pulmonary embolism due to crystallization [see Warnings and Precautions (5.1)].
Hypersensitivity / Allergic Reactions
Advise patients that hypersensitivity/allergic reaction characterized by urticaria, rash, pruritus, and/or various manifestations of angioedema may occur because of apomorphine hydrocloride or any of its excipients including a sulfite (i.e., sodium metabisulfite). Inform patients with a sulfite sensitivity that they may experience various allergic-type reactions, including anaphylactic symptoms and life-threatening asthmatic attacks [see Warnings and Precautions (5.12)]. Advise patients who experience any hypersensitivity/allergic reaction to apomorphine hydrocloride that they should not take apomorphine hydrocloride again [see Contraindications (4)].
Nausea and Vomiting
Advise patients that they may experience severe nausea and/or vomiting and that they should begin taking trimethobenzamide 300 mg orally 3 times per day for 3 days prior to starting apomorphine hydrocloride injections. Advise patients that apomorphine hydrocloride taken with trimethobenzamide may increase the risks for somnolence, dizziness, and falls. Inform patients that their healthcare provider will tell them when trimethobenzamide can be discontinued [see Warnings and Precautions (5.2)].
Falling Asleep Suddenly and Sedation / Sleepiness
Alert patients to the potential sedating effects of apomorphine hydrocloride, including somnolence and falling asleep while engaged in activities of daily living. Instruct patients not to drive a car or engage in other potentially dangerous activities until they have gained sufficient experience with apomorphine hydrocloride to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) occur, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Because of possible additive effects of alcohol use, advise patients to limit their alcohol intake [see Warnings and Precautions (5.3)].
Hypotension / Orthostatic Hypotension
Advise patients that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Hypotension and/or orthostatic symptoms may occur more frequently during initial therapy or with an increase in dose at any time (cases have been seen after months of treatment). Instruct patients to rise slowly after sitting or lying down after taking apomorphine hydrocloride. Inform patients that alcohol and nitroglycerin (and possibly other vasodilators and antihypertensive medications) may potentiate the hypotensive effect of apomorphine hydrocloride [see Warnings and Precautions (5.4)]. Instruct patients ideally to lie down before taking sublingual nitroglycerin and to remain supine and avoid standing for at least 45 minutes after nitroglycerin. Instruct patients taking apomorphine hydrocloride to avoid alcohol while using apomorphine hydrocloride and of the increased hypotensive effects of apomorphine hydrocloride taken with nitroglycerin or by taking apomorphine hydrocloride after alcohol ingestion.
Falls
Alert patients that they may have increased risk for falling when using apomorphine hydrocloride [see Warnings and Precautions (5.5)].
Hallucinations and/or Psychotic-Like Behavior
Inform patients that hallucinations or other manifestations of psychotic-like behavior can occur. Tell patients if they have a major psychotic disorder, ordinarily they should not use apomorphine hydrocloride because of the risk of exacerbating the psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of apomorphine hydrocloride [see Warnings and Precautions (5.6)].
Dyskinesia
Inform patients that apomorphine hydrocloride may cause and/or exacerbate pre-existing dyskinesias [see Warnings and Precautions (5.7)].
Impulse Control / Compulsive Behaviors
Patients and their caregivers should be alerted to the possibility that they may experience intense urges to spend money uncontrollably, intense urges to gamble, increased sexual urges, binge eating and/or other intense urges and the inability to control these urges while taking apomorphine hydrocloride [see Warnings and Precautions (5.8)].
Coronary Events
Inform patients that apomorphine hydrocloride may cause coronary events including angina and myocardial infarction and these outcomes could possibly be related to significant hypotension/orthostatic hypotension [see Warnings and Precautions (5.9)].
QTc Prolongation and Potential for Proarrhythymic Effects
Alert patients that apomorphine hydrocloride may cause QTc prolongation and might produce proarrhythmic effects that could cause torsades de pointes and sudden death. Palpitations and syncope may signal the occurrence of an episode of torsades de pointes [see Warnings and Precautions (5.10)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Advise patients to contact their healthcare provider if they wish to discontinue apomorphine hydrocloride or decrease the dose of apomorphine hydrocloride [see Warnings and Precautions (5.11)].
Priapism
Advise patients that apomorphine hydrocloride may cause prolonged painful erections and that if this occurs they should seek medical attention immediately [see Warnings and Precautions (5.14)].
Injection Site Reactions
Inform patients that injections of apomorphine hydrocloride may result in injection site reactions including bruising, granuloma, and pruritus [see Adverse Reactions (6.1)].
This product's Prescribing Information may have been updated. For current full Prescribing Information, please visit www.usworldmeds.com.
Manufactured by:
Berkshire Sterile Manufacturing, Inc.
Lee, MA
Distributed by:
TruPharma, LLC
Tampa, FL 33609
APOKYN® is a registered trademark of BRITUSWIP.
APOKYN®Pen Instructions for Use
Designed to be used only with
3 mL Apomorphine Hydrochloride Injection Cartridges
For more information, call your specialty pharmacy provider
Apomorphine Hydrochloride injection

APOKYN® Pen
- Apomorphine hydrochloride injection is for under the skin (subcutaneous) injection only.
- Do not inject apomorphine hydrocloride into a vein.
- Do not use the APOKYN® Pen unless you and your care partner have been taught the right way to use it and both of you understand all of the instructions.
- The APOKYN® Pen is for use only with 3 mL apomorphine hydrochloride injection cartridges.
- The APOKYN® Pen is only for use by 1 patient and should not be shared.
| Gray Pen Cap | Gray Pen Body | Pen Needle Unit | Cartridge |
|
|  |  | 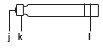 |
|
a. Clip
| b. Black Rod c. White Dose Window d. White Dose Knob e. Teal Injection Button | f. Outer Needle Shield g. Pink Inner Needle Shield h. Pen Needle i. Pink Paper Tab | j. Rubber Septum k. Metal Cap l. Cartridge Plunger |
Read First: Important Safety Information
♦The APOKYN® Pen is a medicine delivery device. It is very important that you or your care partner read this Instructions for Use and follow the instructions for using the APOKYN® Pen correctly to receive the correct apomorphine hydrocloride dose.
♦Always perform a flow check (prime) before every injection and after loading a new cartridge.
♦The liquid in the apomorphine hydrocloride cartridge can cause irritation if it gets on your skin or in your eyes. Flush your eyes with cold water and wash the liquid off your skin right away if this happens.
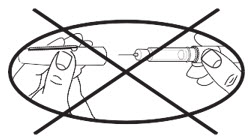
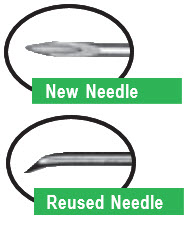
♦The BD pen needle unit is sterile. Avoid contaminating the needle after opening. Do not place it on a surface or touch other items with the needle.
♦Do not dial the dose or try to correct a dialing error with the pen needle in the skin. You could receive the wrong dose.
♦Be careful when removing the needle. Accidental needle sticks can transmit serious infections.
Never store or carry the APOKYN® Pen with a pen needle attached.
|
| Storing or carrying the APOKYN® Pen with a pen needle attached may let: ♦Air enter the cartridge♦Medicine leak outThis can affect your apomorphine hydrocloride dose. |
The APOKYN® Pen should only be used with pen needles (29G × 1/2"). These needles are available through your specialty pharmacy provider or your local pharmacy.
|
| You must use a new, sterile BD pen needle with each injection. | |
| Magnification: 50× |
How to Use the APOKYN® Pen
|
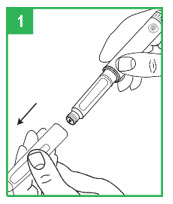
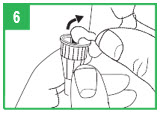
| 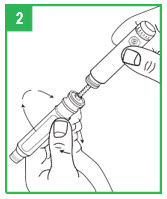 | Preparing the apomorphine hydrocloride Pen for Cartridge Loading Step 1. Remove the gray pen cap by pulling it straight off. Step 2. Unscrew the teal cartridge holder from the gray pen body by turning it clockwise. |
|
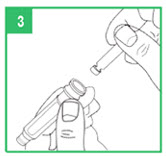
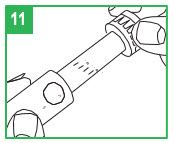
| Loading a Cartridge Step 3. Only use apomorphine hydrocloride that is clear and colorless. Do not use an apomorphine hydrocloride cartridge that contains medicine that is cloudy, green, or contains particles. Call your specialty pharmacy provider for replacement cartridges. Insert the apomorphine hydrocloride cartridge, metal cap first, into the teal cartridge holder. Step 4. Lower the gray pen body onto the teal cartridge holder so that the black rod presses against the cartridge plunger. Screw the teal cartridge holder onto the gray pen body. Tighten the pieces by turning the teal cartridge holder clockwise until no gap remains and 1 of the white arrows line up with the white marker on the gray pen body. |
|
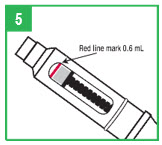
| Step 5. If you already have a cartridge in the pen and have used the pen, you should check the cartridge through the window in the teal cartridge holder to make sure there is enough apomorphine hydrocloride solution in the cartridge to provide your next dose. If the gray cartridge plunger has reached the red line on the cartridge, remove the cartridge and insert a new cartridge into the pen before attaching the pen needle and preparing the dose. |
|
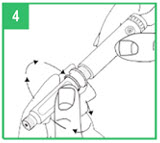
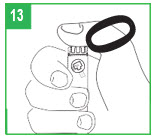
| Attaching the Pen Needle Step 6. Remove the pink paper tab from the back of a new pen needle. Use a new needle for each injection. Never reuse needles. Step 7. Holding the apomorphine hydrocloride Pen by the teal cartridge holder, push the pen needle unit onto the pen. Screw the threaded hub of the pen needle onto the teal cartridge holder counter-clockwise. When the needle unit is attached, remove the outer shield that protects the needle with a gentle pull. Save the outer shield. You will use it to remove the needle from the pen after the injection is finished. Do not remove the inner needle shield at this time. The needle is sterile and must stay clean. After opening, do not place the needle on a surface or let it touch anything. |
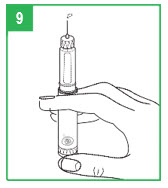
Preparing (Priming) the APOKYN® Pen for Use
| IMPORTANT – Prior to each injection, it is important that the APOKYN® Pen be properly primed. |
| For a new apomorphine hydrocloride cartridge (1 that has not been used before), repeat the priming procedure described on the next page (Steps 8-9) 3 or 4 times to make sure all the air has been removed from the needle and cartridge. |
| For an apomorphine hydrocloride cartridge you have used before (1 that has been previously primed), repeat the priming procedure described on the next page (Steps 8-9) 1 time to make sure all the air has been removed from the needle and cartridge. |
|
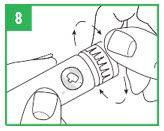
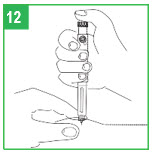
| Step 8. You must prepare (prime) the apomorphine hydrocloride Pen for use before injecting the medicine. To prime the APOKYN® Pen, set the dose by turning the dose knob to 0.1 mL. This is important so you can get rid of any air bubbles in the cartridge. Step 9. Remove the inner needle shield. Remember, do not let the needle touch any- thing. With the needle pointing up, firmly push the injection button in as far as it will go and hold for at least 5 seconds. A small stream of medicine must come out of the end of the needle. If it does not, reset the dose by repeating Step 8. Repeat these steps (Steps 8-9) until a small stream of medicine comes out the end of the needle. When medicine comes out of the end of the needle, the APOKYN® Pen is primed for injection and ready to use. Apomorphine hydrocloride medicine can cause staining to fabric and other surfaces it touches. Be careful where you prime the APOKYN® Pen. |
|
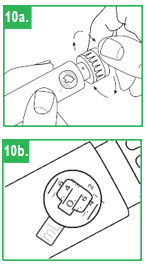
| Setting the Dose Step 10. To set the dose, turn the white dose knob until the correct dose (number of mLs) is shown in the window. The dose will appear as a red number between two black lines that will line up next to the letters "mL" on the pen body. Make sure the correct number (dose) appears in the window. |
|
| Step 11. Dose Correction. If you turn the dose knob past your dose, do not dial backwards. If you dial backwards, apomorphine hydrocloride will be pushed through the needle and you will lose medicine. Continue to turn the dial until it is fully turned. Press the injection button fully. This will reset the dial to zero without pushing medicine out of the needle. Repeat Step 10 to redial your dose. |
|
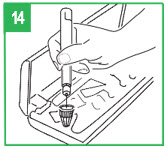
| Giving the Injection Step 12. apomorphine hydrocloride is only for injection under the skin (subcutaneous injection). Choose an injection site on your stomach area, upper arm or upper leg. Change the site with each injection. Do not inject apomorphine hydrocloride into skin that is red or sore. Clean the site with an alcohol swab and allow to air dry. Pinch about an inch of skin and fat tissue of your injection site between your thumb and forefinger. With the other hand, insert the needle all the way into the pinched skin. Step 13. Fully push the teal injection button on the APOKYN® Pen. A clicking sound will be heard while the dose is injected. Push the injection button firmly for 5 seconds. Remove the needle from your skin. If medicine keeps dripping from the needle, keep the needle in the skin longer the next time you inject apomorphine hydrocloride. |
| IMPORTANT – If you set your dose and cannot depress the teal injection button, the cartridge is empty. Remove the pen needle and cartridge and prepare the pen as described in Steps 2-9 with a new cartridge. Set the dose and give the injection. |
| If you set your dose and the injection button stops before you receive a complete dose, note the number in the window, remove the pen needle and cartridge, and prepare the pen as described in Steps 2-9 with a new cartridge. Set the dose to the number that last appeared in the window and administer the injection. This completes the dose. |
| Before attempting to replace a cartridge, be sure that a needle unit is not attached to the APOKYN® Pen. |
|
| Removing the Pen Needle Step 14. Carefully replace the outer needle shield. Be careful to avoid a needle-stick. Place the outer needle shield in the notch located on the far left side of your carrying case. The opening of the needle shield should be pointing up. Carefully insert the needle (attached to the pen) into the opening of the shield. Without holding onto the shield, push down firmly. |
|
| Step 15. Hold the pen by the teal cartridge holder and unscrew the pen needle from the cartridge holder. Recap the pen. Never recap the pen with a needle attached. Safely dispose of used pen needles in a "sharps" container. Your specialty pharmacy provider will provide you with a "sharps" container. Do not throw used needles in a trash can. |
Storage Information
–Store apomorphine hydrocloride cartridges at room temperature, 68°F to 77°F (20°C to 25°C)
Excursions permitted between 59 to 86°F (15 to 30°C) [See USP Controlled Room Temperature]
Proper Disposal
♦Put your used needles and syringes in an FDA-cleared sharps disposal container right away after use. Do not throw away (dispose of) loose needles and syringes in your household trash.
♦If you do not have an FDA-cleared sharps disposal container, you may use a household container that is:–made of a heavy-duty plastic,–able to be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,–upright and stable during use,–leak-resistant–properly labeled to warn of hazardous waste inside the container.
♦When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
♦Do not dispose of your used sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
Care and Storage
The APOKYN® Pen can now be stored in its carrying case. Never store or carry the APOKYN® Pen with a pen needle attached.
You must store and care for your pen the right way:
♦Avoid exposure to dust, moisture, and cold or hot temperatures.
♦Never wash the pen in water or use strong disinfectants. Only a clean, damp cloth should be used for cleaning.
♦Do not try to repair the pen if it is damaged or if you cannot solve a problem shown in the following "Troubleshooting" section.
♦Do not use pen for more than 1 year after the first use or after the expiration date on the carton.
For more information, call your specialty pharmacy provider.
Troubleshooting
| PROBLEM | POSSIBLE CAUSE | HOW TO FIX THE PROBLEM |
|---|---|---|
| For more information, call your specialty pharmacy provider. | ||
| No medicine comes out of the pen (the dosage dial moves freely, but no click is heard). | The pen is in dose correction mode. | Push the injection button all the way in so the dial returns to zero. |
| The dosage dial does not return to zero during an injection. | The cartridge is empty. | Replace the cartridge as described in Steps 3-5. |
| The pen needle is clogged. | Replace the pen needle as described in Steps 14 & 6-7. | |
| The dosage dial does not turn easily. | Dust or dirt is on the pen. | Turn the dial past the highest setting on the pen. Wipe all exposed pen surfaces with a clean, damp cloth. |
| Pen does not close. | Cartridge is inserted incorrectly. | Remove cartridge and reload it. See Steps 3 - 4. |
| Injection button will not depress. | Cartridge is empty. | Replace cartridge. See Steps 3-5. |
| Injection button stops before a complete dose is delivered. | Not enough medication in cartridge to complete the dose. | Replace cartridge. See Steps 3-5. |
| Pen does not work. | Mechanical failure. | Replace pen. Call your specialty pharmacy provider. |
| Dose numbers and/or white markers wear off. | Repeated use over extended period of time. | Replace pen. Call your specialty pharmacy provider. |
| Too much force needed to depress injection button. | Defective cartridge. | Replace cartridge. See Steps 3-5. |
| Unable to read the dose numbers through the dose window. | Incorrect cleaning or improper handling. | Replace pen. Call your specialty pharmacy provider. |
Patient Information
Apomorphine Hydrochloride Injection
(a”poe mor’ feen hye” droe klor’ ide)
Read this Patient Information before you start using apomorphine hydrocloride injection and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Use only with the APOKYN® Pen for subcutaneous (under the skin) administration.
What is apomorphine hydrocloride injection?
Apomorphine hydrocloride injection is a prescription medicine used to treat acute, intermittent episodes of poor mobility called "off " episodes (end-of-dose wearing "off " or unpredictable "on -off " episodes) in people with advanced Parkinson's disease (PD).
It is not known if apomorphine hydrocloride is safe and effective in children.
Who should not take apomorphine hydrocloride injection?
Do not take apomorphine hydrocloride injection if you are:
- taking certain medicines to treat nausea called 5HT3 antagonists including, ondansetron, granisetron, dolasetron, palonosetron, and alosetron. People taking ondansetron together with apomorphine, the active ingredient in apomorphine hydrocloride injection, have had very low blood pressure and lost consciousness or "blacked out."
- allergic to apomorphine hydrochloride injection or to any of the ingredients in apomorphine hydrocloride injection and experience hives, itching, rash, or swelling (e.g., eyes, tongue, etc.). apomorphine hydrocloride injection also contains a sulfite called sodium metabisulfite. Sulfites can cause severe, life-threatening allergic reactions in some people. An allergy to sulfites is not the same as an allergy to sulfa. People with asthma are more likely to be allergic to sulfites. Call your healthcare provider if you have hives, itching, rash, swelling of the eyes, tongue, lips, chest pain, trouble breathing or swallowing. See the end of this leaflet for a complete list of ingredients in apomorphine hydrocloride injection.
What should I tell my healthcare provider before taking apomorphine hydrocloride injection?
Before you start using apomorphine hydrocloride injection, tell your healthcare provider if you:
- have difficulty staying awake during the daytime
- have dizziness
- have fainting spells
- have low blood pressure
- have asthma
- are allergic to any medicines containing sulfites
- have liver problems
- have kidney problems
- have heart problems
- have had a stroke or other brain problems
- have a mental problem called a major psychotic disorder
- drink alcohol
- are pregnant or plan to become pregnant. It is not known if apomorphine hydrocloride injection will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if apomorphine hydrocloride passes into your breast milk. You and your healthcare provider should decide if you will take apomorphine hydrocloride injection or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Using apomorphine hydrocloride injection with certain other medicines may affect each other. Using apomorphine hydrocloride injection with other medicines can cause serious side effects.
- If you take nitroglycerin under your tongue while using apomorphine hydrocloride injection, your blood pressure may decrease and cause dizziness. After taking nitroglycerin, you should lie down and try to continue lying down for at least 45 minutes. You should avoid standing for 45 minutes after taking nitroglycerin.
Know the medicines you take. Keep a list of them to show your healthcare provider or pharmacist when you get a new medicine.
How should I use apomorphine hydrocloride injection?
- Read the Instructions for Use starting on page 6 of this leaflet for specific information about the right way to use apomorphine hydrocloride injection.
- Use apomorphine hydrocloride injection exactly as your healthcare provider tells you to use it.
- Your healthcare provider will tell you how much apomorphine hydrocloride injection to use and teach you the right way to use it.
- Your healthcare provider may change your dose if needed.
- Do not change your dose of apomorphine hydrocloride injection or use it more often than prescribed unless your healthcare provider has told you to.
- Do not give another dose of apomorphine hydrocloride injection sooner than 2 hours after the last dose.
- Your healthcare provider will prescribe apomorphine hydrocloride injection that comes in prefilled glass cartridges, for single-patient-use, that are used with a special pen injector.
- Your APOKYN® pen is dosed in milliliters (mL), not milligrams (mg). Make sure your prescription tells you how many milliliters (mL) to use.
- Inject apomorphine hydrocloride injection under your skin (subcutaneously). Do not inject apomorphine hydrocloride injection into a vein.
- Keep a record of how much apomorphine hydrocloride injection you have used each time you inject or your care partner gives you an injection.
- Use a new needle with each injection. Never reuse a needle.
- apomorphine hydrocloride injection is a clear and colorless liquid. Do not use apomorphine hydrocloride injection if it appears cloudy, colored, or to contain particles, and call your pharmacist.
- Your healthcare provider may prescribe another medicine called an antiemetic to take while you are using apomorphine hydrocloride injection. Antiemetic medicines help to decrease the symptoms of nausea and vomiting that can happen with apomorphine hydrocloride injection.
- If you take too much apomorphine hydrocloride injection, call your healthcare provider. If you experience severe or serious side effects such as chest pain or prolonged erection lasting more than 4 hours, go to the nearest hospital emergency room.
What should I avoid while using apomorphine hydrocloride injection?
- Do not drink alcohol while you are using apomorphine hydrocloride injection. It can increase your chance of developing serious side effects.
- Do not take medicines that make you sleepy while you are using apomorphine hydrocloride injection.
- Do not drive, operate machinery, or do other dangerous activities until you know how apomorphine hydrocloride injection affects you.
- Do not change your body position too fast. Get up slowly from sitting or lying. apomorphine hydrocloride injection can lower your blood pressure and cause dizziness or fainting.
What are the possible side effects of apomorphine hydrocloride injection?
Apomorphine hydrocloride injection may cause serious side effects. Call your healthcare provider right away if you have any of the serious side effects, including:
- allergic reaction. An allergic reaction with side effects of hives, itching, rash, swelling (e.g., eyes, tongue, etc.); trouble breathing and/or swallowing may occur after injecting apomorphine hydrocloride injection.
- blood clots. Injecting apomorphine hydrocloride injection into a vein (intravenous) can cause blood clots. Do not inject apomorphine hydrocloride injection in your vein.
- nausea and vomiting. Severe nausea and vomiting can happen with apomorphine hydrocloride injection. Your healthcare provider may prescribe a medicine called trimethobenzamide (Tigan®) to help prevent nausea and vomiting. Some patients can stop taking Tigan after using apomorphine hydrocloride injection for several months. Some patients may need to keep taking Tigan to help prevent nausea and vomiting. Talk to your healthcare provider before you stop taking Tigan.
- sleepiness or falling asleep during the day. Some people treated with apomorphine hydrocloride injection may get sleepy during the day or fall asleep without warning while doing everyday activities such as talking, eating, or driving a car.
- dizziness. apomorphine hydrocloride injection can lower your blood pressure and cause dizziness. Dizziness can happen when apomorphine hydrocloride treatment is started or when the apomorphine hydrocloride injection dose is increased. Do not get up too fast from sitting or after lying down, especially if you have been sitting or lying down for a long period of time.
- falls. The changes that can happen with Parkinson's disease (PD), and the effects of some PD medicines, can increase the risk of falling. apomorphine hydrocloride may also increase your risk of falling.
- hallucinations or psychotic-like behavior. apomorphine hydrocloride injection can cause or worsen psychotic-like behavior including hallucinations (seeing or hearing things that are not real), confusion, excessive suspicion, aggressive behavior, agitation, delusional beliefs (believing things that are not real), and disorganized thinking.
- sudden uncontrolled movements (dyskinesias). Some people with PD may get sudden, uncontrolled movements after treatment with some PD medicines. apomorphine hydrocloride injection can cause or make dyskinesias worse.
- intense urges. Some people with PD have reported new or increased gambling urges, increased sexual urges, and other intense urges, while taking PD medicines, including apomorphine hydrocloride injection.
- heart problems. If you have shortness of breath, fast heartbeat, or chest pain while taking apomorphine hydrocloride injection, call your healthcare provider or get emergency help right away.
- serious heart rhythm changes (QT prolongation). Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat), or if you faint.
- injection site problems. Bruising, swelling, and itching can happen at the site where you inject apomorphine hydrocloride injection.
- fever and confusion. This can happen in some people when their PD medicine is stopped or there is a fast decrease in the dose of their PD medicine.
- tissue changes (fibrotic complications). Some people have had changes in the tissues of their pelvis, lungs, and heart valves when taking medicines called nonergot derived dopamine agonists like apomorphine hydrocloride injection.
- prolonged painful erections (priaprism). apomorphine hydrocloride injection may cause prolonged, painful erections in some people. If you have an erection that lasts more than 4 hours you should call your healthcare provider or go to the nearest hospital emergency room right away.
- swelling of ankles/legs. apomorphine hydrocloride injection may cause swelling, especially in the ankles or legs. Tell your healthcare provider if you notice any swelling.
Other common side effects of apomorphine hydrocloride injection include:
- yawning
- runny nose
- confusion
- swelling of your hands, arms, legs, and feet
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of apomorphine hydrocloride injection. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to TruPharma, LLC at 877-541-5504.
How should I store apomorphine hydrocloride injection?
- Store apomorphine hydrocloride injection at room temperature, 68°F to 77°F (20°C to 25°C).
- Safely throw away medicine that is out of date or no longer needed.
Keep apomorphine hydrocloride injection and all medicines out of the reach of children.
General information about the safe and effective use of apomorphine hydrocloride injection.
Medicines are sometimes prescribed for purposes other than those listed in a patient information leaflet. Do not use apomorphine hydrocloride injection for a condition for which it was not prescribed. Do not give apomorphine hydrocloride to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information leaflet summarizes the most important information about apomorphine hydrocloride injection. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about apomorphine hydrocloride injection that is written for health professionals.
What are the ingredients in apomorphine hydrocloride injection?
Active ingredient: apomorphine hydrochloride, USP
Inactive ingredients: sodium metabisulfite, NF, benzyl alcohol, NF, water for injection, USP. It may also contain sodium hydroxide, NF and/or hydrochloric acid, NF.
This Patient Information and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured by:
Berkshire Sterile Manufacturing, Inc.
Lee, MA
Distributed by:
TruPharma, LLC
Tampa, FL 33609
APOKYN® is a registered trademark of BRITUSWIP.
BD and BD Logo are trademarks of Becton, Dickinson and Company.
PRINCIPAL DISPLAY PANEL - 30 mg/3 mL Cartridge Carton
NDC 52817-720-05
5 × 3 mL cartridges for single-patient-use
Rx only
Apomorphine Hydrochloride Injection
30 mg/3 mL (10 mg/mL)
For use only with the APOKYN® Pen for
subcutaneous administration.
Not for Intravenous Use.
Single-patient-use with pen injector