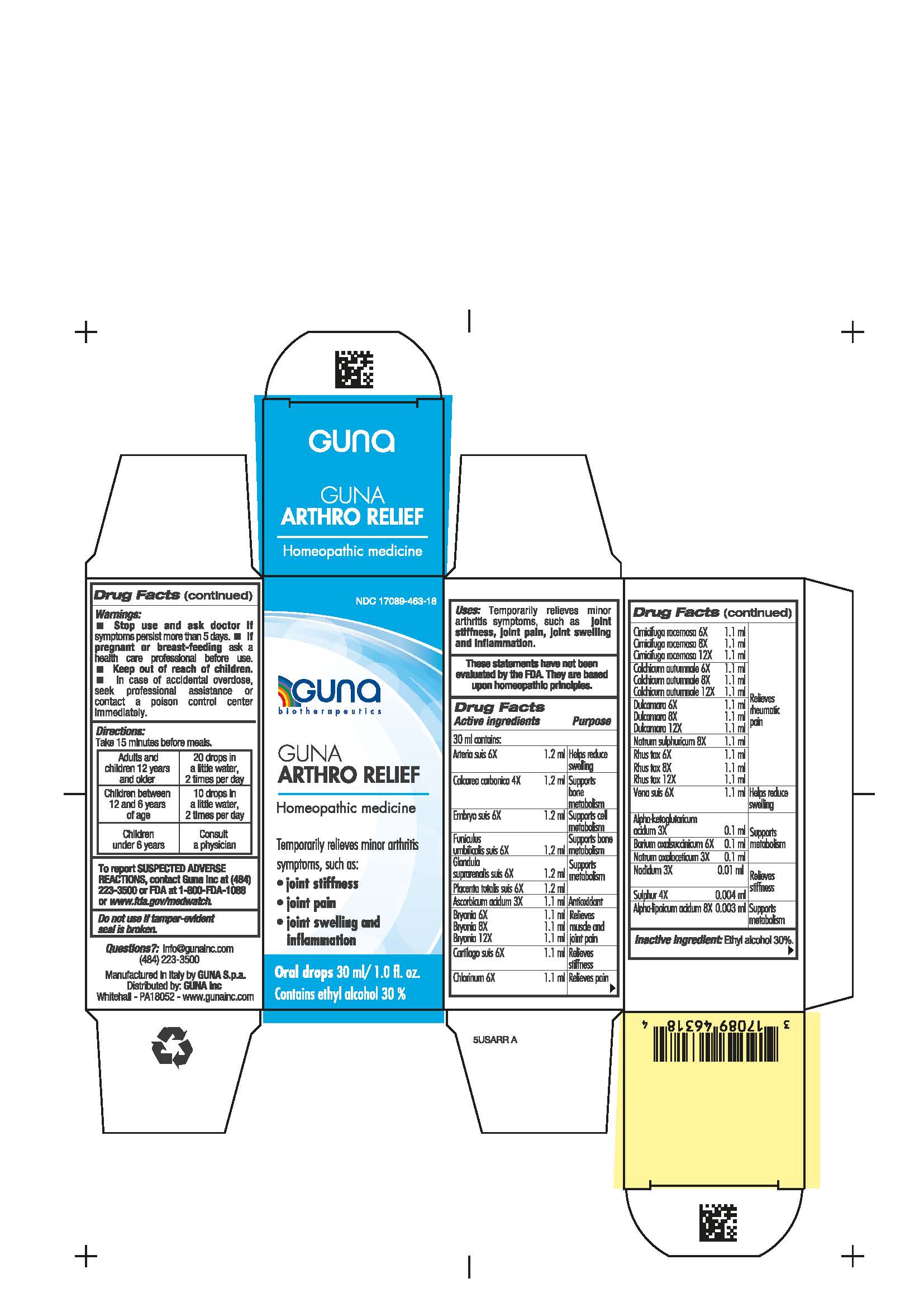
DIRECTIONS
Adults and children 12 years and older 20 drops in a little water 2 times per day
Children between 12 years and 6 years of age 10 drops in a little water 2 times per day
Children under 6 years consult a physician
WARNINGS
- Stop use and ask doctor if symptoms persist more than 5 days.
- If pregnant or breast-feeding ask a health care professional before use.
- Keep out of reach of children. In case of overdose, seek professional assistance or contact a Poison Control Center immediately.
- Contains ethyl alcohol 30%
USES
Temporarily relieves minor arthritic symptoms, such as:
- Joint stiffness
- Joint pain
- Joint swelling and inflammation
ACTIVE INGREDIENTS/PURPOSE
Arteria suis 6X Helps reduce swelling
Calcarea carbonica 4X Supports bone metabolism
Embryo suis 6X Supports cell metabolism
Funiculus umbilicalis suis 6X Supports bone metabolism
Glandula suprarenalis suis 6X Supports metabolism
Placenta totalis suis 6X Supports metabolism
Ascorbicum acidum 3X Antioxidant
Bryonia alba 6X, 8X, 12X Relieves muscle and joint pain
Cartilago suis 6X Relieves stiffness
Chlorinum 6X Relieves pain
Cimicifuga racemosa 6X, 8X, 12X Relieves rheumatic pain
Colchicum autumnale 6X, 8X, 12X Relieves rheumatic pain
Dulcamara 6X, 8X, 12X Relieves rheumatic pain
Natrum sulphuricum 8X Relieves rheumatic pain
Rhus toxicodendron 6X, 8X, 12X Relieves rheumatic pain
Vena suis 6X Helps reduce swelling
Alpha-ketoglutaricum acidum 3X Supports metabolism
Barium oxalsuccinicum 6X Supports metabolism
Natrum oxalaceticum 3X Supports metabolism
Nadidum 3X Relieves stiffness
Sulphur 4X Relieves stiffness
Alpha-lipoicum acidum 8X Supports metabolism