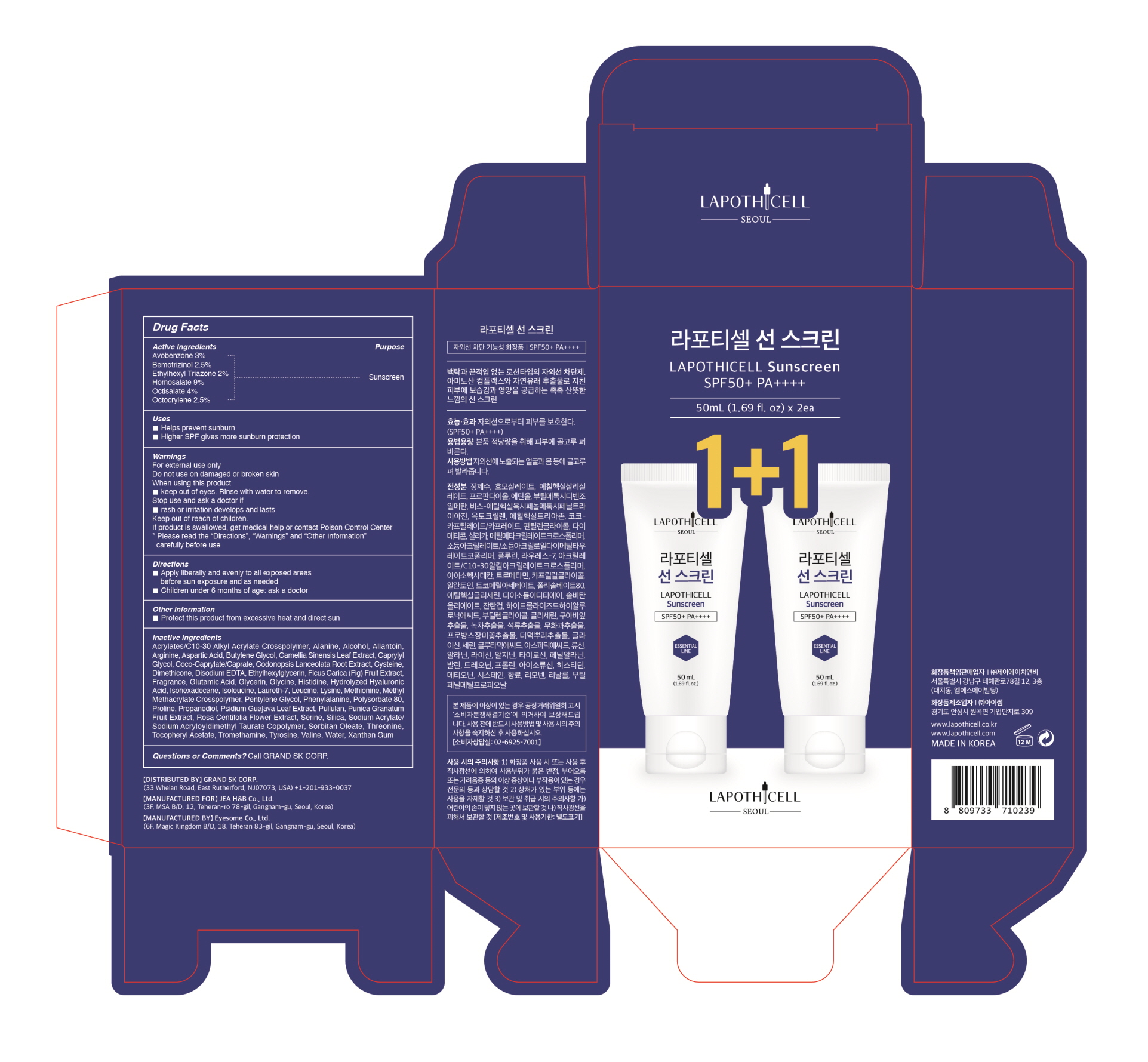
For external use only
Do not use on damaged or broken skin
When using this product
*Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if
*rash or irritation develops and lasts
Keep our of reach of children.
If product is swallowed, get medical help or contact Poison Control Center
*Please read the "Directions", "Warnings", and "Other Information" carefully before use
Keep out of reach of children.
If product is swallowed, get medical
Help or contact a poison Control Center right away.
Apply liberally and evenly to all exposed areas before sun exposure and as needed
Children under 6 months of age: ask a doctor
Water, Propanediol, Alcohol, Coco-Caprylate/Caprate, Pentylene Glycol, Dimethicone, Silica, Methyl Methacrylate Crosspolymer, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Pullulan, Laureth-7, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Isohexadecane, Tromethamine, Caprylyl Glycol, Allantoin, Tocopheryl Acetate, Polysorbate 80, Ethylhexylglycerin, Disodium EDTA, Sorbitan Oleate, Xanthan Gum, Hydrolyzed Hyaluronic Acid, Butylene Glycol, Glycerin, Psidium Guajava Leaf Extract, Camellia Sinensis Leaf Extract, Punica Granatum Fruit Extract, Ficus Carica (Fig) Fruit Extract, Rosa Centifolia Flower Extract, Codonopsis Lanceolata Root Extract, Glycine, Serine, Glutamic Acid, Aspartic Acid, Leucine, Alanine, Lysine, Arginine, Tyrosine, Phenylalanine, Valine, Threonine, Proline, Isoleucine, Histidine, Methionine, Cysteine, Fragrance, Limonene, Linalool, Butylphenyl Methylpropional