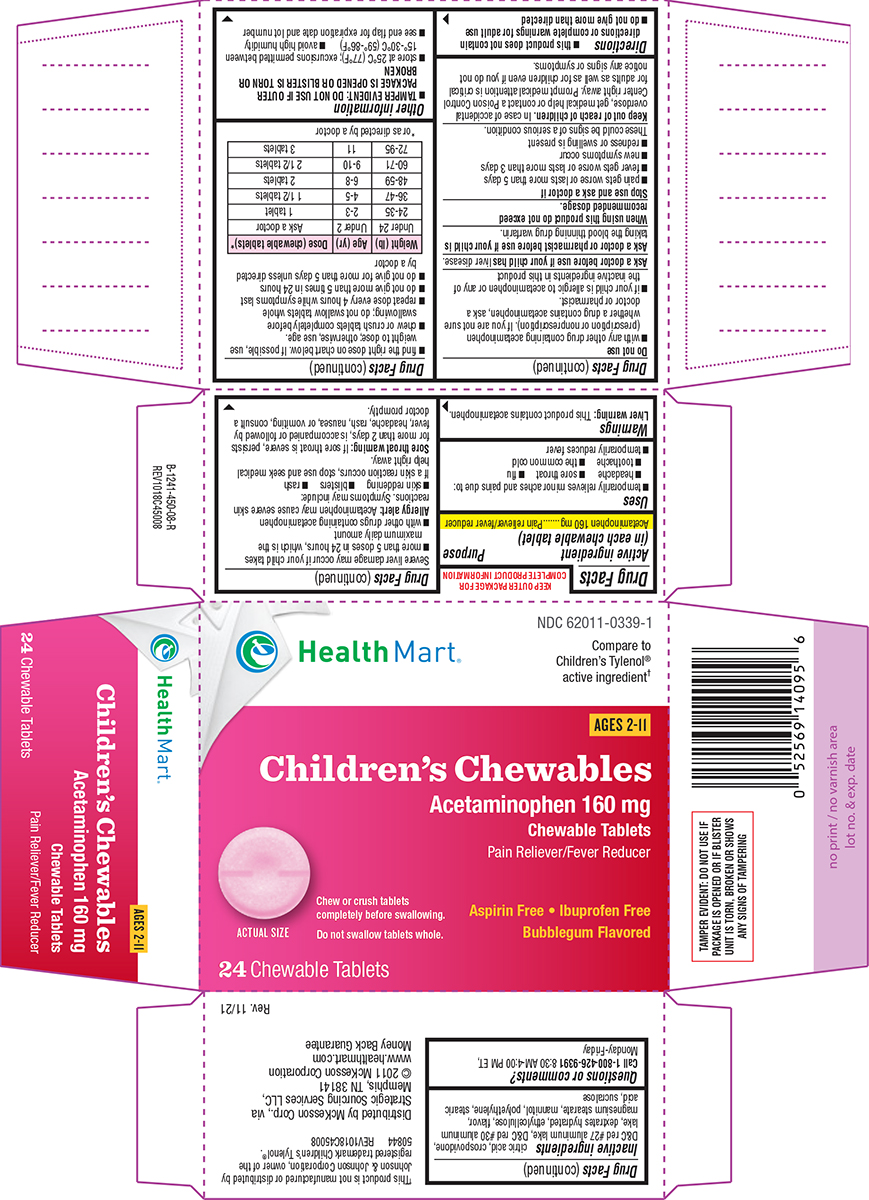
Uses
- temporarily relieves minor aches and pains due to:
- headache
- sore throat
- flu
- toothache
- the common cold
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
| Weight (lb) | Age (yr) | Dose (chewable tablets)* |
| Under 24 | Under 2 | Ask a doctor |
| 24-35 | 2-3 | 1 tablet |
| 36-47 | 4-5 | 1 1/2 tablets |
| 48-59 | 6-8 | 2 tablets |
| 60-71 | 9-10 | 2 1/2 tablets |
| 72-95 | 11 | 3 tablets |
*or as directed by a doctor
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- avoid high humidity
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
citric acid, crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates hydrated, ethylcellulose, flavor, magnesium stearate, mannitol, polyethylene, stearic acid, sucralose
Principal Display Panel
Health Mart®
NDC 62011-0339-1
Compare to
Children's Tylenol®
active ingredient†
AGES 2-11
Children's Chewables
Acetaminophen 160 mg
Chewable Tablets
Pain Reliever/Fever Reducer
Aspirin Free • Ibuprofen Free
Bubblegum Flavored
Chew or crush tablets
completely before swallowing.
Do not swallow tablets whole.
Actual Size
24 Chewable Tablets
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
†This product is not manufactured or distributed by
Johnson & Johnson Corporation, owner of the
registered trademark Children's Tylenol®.
50844 REV1018C45008
Distributed by McKesson Corp., via
Strategic Sourcing Services LLC,
Memphis, TN 38141
© 2011 McKesson Corporation
www.healthmart.com
Money Back Guarantee
Health mart 44-450