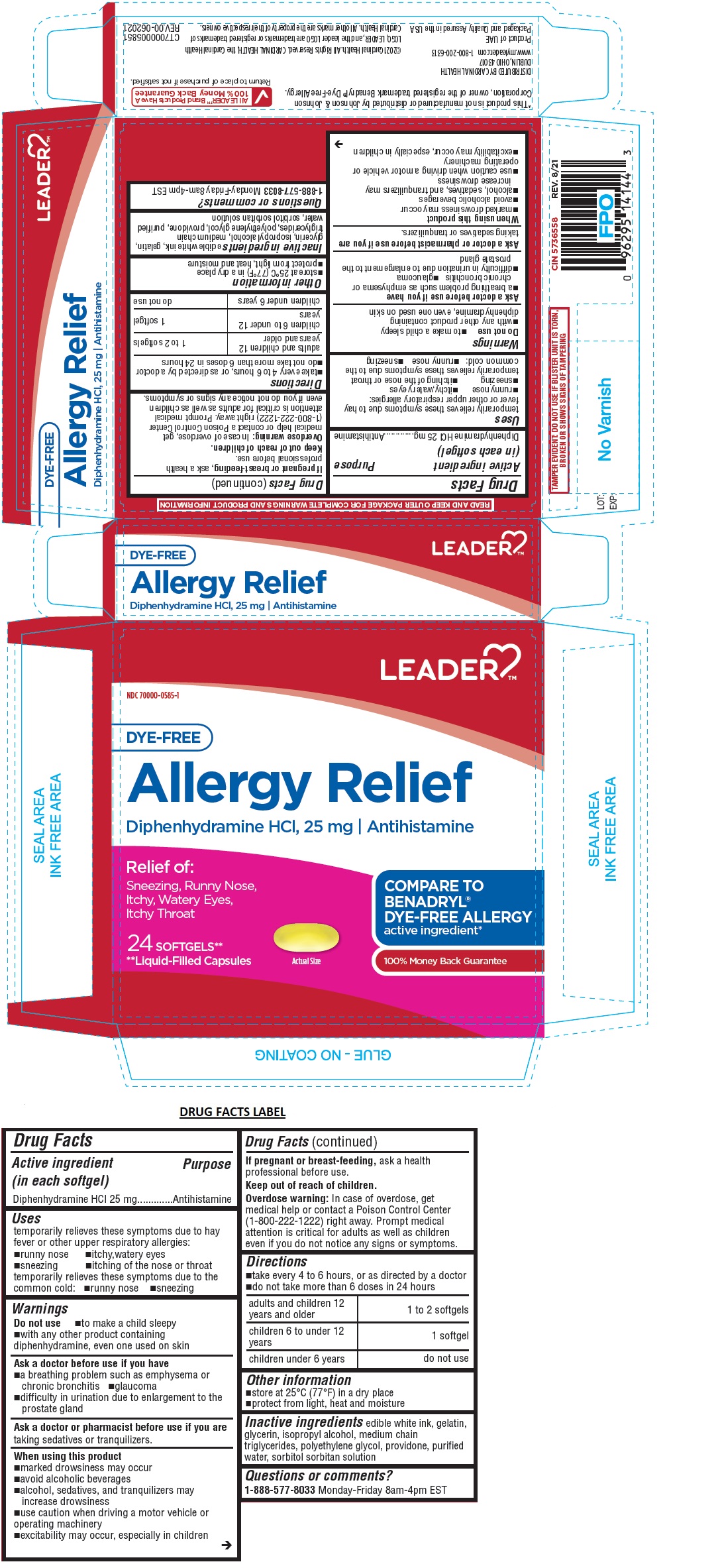
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
•runny nose •itchy,watery eyes •sneezing •itching of the nose or throat temporarily relieves these symptoms due to the common cold: •runny nose •sneezing
Warnings
Do not use •to make a child sleepy
•with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
•a breathing problem such as emphysema or chronic bronchitis •glaucoma
•difficulty in urination due to enlargement to the prostate gland
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers.
When using this product
•marked drowsiness may occur
•avoid alcoholic beverages
•alcohol, sedatives, and tranquilizers may increase drowsiness
•use caution when driving a motor vehicle or operating machinery
•excitability may occur, especially in children
If pregnant or breast-feeding, ask a health professional before use
Directions
•take every 4 to 6 hours, or as directed by a doctor
•do not take more than 6 doses in 24 hours
| adults and children 12 years and older | 1 to 2 softgels |
| children 6 to under 12 years | 1 softgel |
| children under 6 years | do not use |
Inactive ingredients
edible white ink, gelatin, glycerin, isopropyl alcohol, medium chain triglycerides, polyethylene glycol, povidone, purified water, sorbitol sorbitan solution
LEADER ™
DYE-FREE
Relief of: Sneezing, Runny Nose, Itchy, Watery Eyes, Itchy Throat
COMPARE TO BENADRYL® DYE-FREE ALLERGY active ingredient*
100% Money Back Guarantee
READ AND KEEP OUTER PACKAGE FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Benadryl® Dye-Free Allergy.
All LEADERTM Brand Products Have A 100% Money Back Guarantee
Return to place of purchase if not satisfied.
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
Product of UAE
Packaged and Quality Assured in the USA
©2021 Cardinal Health. All Rights Reserved. CARDINAL HEALTH, the Cardinal Health LOGO, LEADER, and the Leader LOGO are trademarks or registered trademarks of Cardinal Health. All other marks are the property of their respective owners.
TAMPER EVIDENT: DO NOT USE IF BLISTER UNIT IS TORN, BROKEN OR SHOWS SIGNS OF TAMPERING