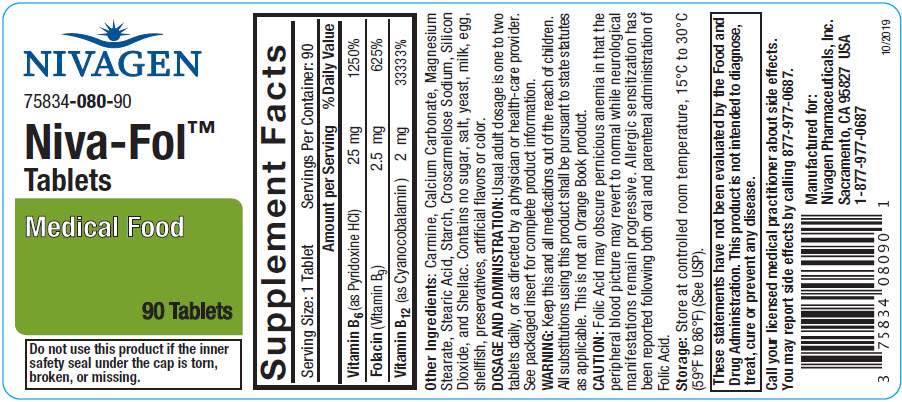
Each Niva-Fol™ Tablet contains:
| Vitamin B6 (as Pyridoxine HCl) | 25 mg |
| Folacin (Folic Acid) | 2.5 mg |
| Vitamin B12 (as Cyanocobalamin) | 2 mg |
Other Ingredients:
Carmine, Calcium Carbonate, Magnesium Stearate, Stearic Acid, Starch, Croscarmellose Sodium, Silicon Dioxide, and Shellac. Contains no sugar, salt, yeast, milk, egg, shellfish, preservatives, artificial flavors or color.
INDICATIONS AND USAGE
For the dietary management of individuals with distinct nutritional needs under a physician or health-care provider's supervision for hyperhomocysteinemia; with particular emphasis for individuals with or at risk for atherosclerotic vascular disease in the coronary1, peripheral2, or cerebral3 vessels, or vitamin B12 deficiency4. Niva-Fol™ Tablets are labeled as a medical food intended for use under active and ongoing medical supervision requiring medical care on a recurring basis for, among other things, instructions on the use of the medical food.
MEDICAL FOODS
Medical foods are Intended for the dietary management of a patient who, because of therapeutic or chronic medical needs, has limited or impaired capacity to ingest, digest, absorb, or metabolize ordinary foodstuffs or certain nutrients, or who has other special medically determined nutrient requirements, the dietary management of which cannot be achieved by the modification of a normal diet alone. Although a medical-food product is intended for use under the active and ongoing medical supervision, FDA does not require a prescription.
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent any disease. |
PRECAUTIONS
Folacin (folic acid) when administered as a single agent in doses above 0.1 mg daily, may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive. The 2 mg of cyanocobalamin contained in Niva-Fol™ Tablets has been shown to provide an adequate amount of cyanocobalamin to address this precaution5. Unmetabolized folic acid has been shown in one study of 105 postmenopausal women (50-75 yrs) to have the potential to reduce natural killer cells' cytotoxicity, which may result in an impaired immune response6.
Cyanocobalamin should not be used in those with Leber's optic atrophy. Decreased levels of B12 have been associated with reduced ability to detoxify the cyanide in exposed individuals and cyanocobalamin may increase the risk of irreversible neurological damage from optic atrophy in those affected with the disorder. Hydroxocobalamin can aid in the detoxification of cyanide. This form of B12 is an acceptable form for B12 supplementation in those with this disorder.
Pregnant women and nursing mothers should only use 12 microgram doses of B12 (cyanocobalamin) from nutritional supplements. Doses higher than this should only be recommended by your physician. Administration of doses of vitamin B12 greater than 10 micrograms daily may produce a hematological response in those with anemia secondary to folate deficiency.
If pregnant, or planning to become pregnant or are currently breast-feeding please contact your physician, or health-care provider before using or continuing use.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folacin (folic acid). Paresthesia, somnolence, nausea and headaches have been reported with pyridoxine. Mild transient diarrhea, polycythemia vera, itching, transitory exanthema, and the feeling of swelling of the entire body has been associated with cyanocobalamin.
CONTRAINDICATIONS
Known hypersensitivity to any of the components in the product is a contraindication.
DRUG INTERACTIONS
Pyridoxine supplements should not be given to patients receiving the drug levodopa, because the action of levodopa is antagonized by pyridoxine. However, pyridoxine may be used concurrently in patients receiving a preparation containing both carbidopa and levodopa. Concurrent use of phenytoin and folacin (folic acid) may result in decreased phenytoin effectiveness.
PATIENT INFORMATION
Niva-Fol™ Tablets are for use only under the direction and supervision of a licensed physician or health-care provider.
DOSAGE AND ADMINISTRATION
Usual adult dosage is one to two tablets daily, or as directed by a physician or health-care provider.
HOW SUPPLIED
Niva-Fol™ Tablets are available as an oval, coated tablet, debossed "N080". Supplied in bottles of 90 Tablets, 75834-080-90
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
See USP Controlled Room Temperature.
Protect from light and moisture.
Dispense in a tight, light-resistant container with a child-resistant closure as defined in the USP/NF.
WARNING: KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Medical Food
All substitutions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
To report a serious adverse event contact: 1-877-977-0687
Manufactured for:
Nivagen Pharmaceuticals, Inc.
Sacramento, CA 95827
References
1, 2 Eikelboom JW, Lonn Eva, Genest Jr Jaques, Hankey Graeme, Yusuf Salim: Homocysteine and Cardiovascular Disease: A Critical Review of the Epidemiologic Evidence. Ann Intern Med. 1999; 131:363-375.
3 The Homocysteine Studies Collaboration: Homocysteine and Risk of lschemic Heart Disease and Stroke. JAMA 2002; Vol 288, No. 16:2015-2022.
4 Refsum Helga, Smith A. David, Ueland Per M, Nexo Ebba, Clarke Robert, McPartlin Joseph, Johnston Carole, Engbaek Frode, Schneede Jorn, McPartlin Catherine, and Scott John M.: Facts and Recommendations about Total Homocysteine Determinations: An Expert Opinion. Clinical Chemistry 2004; 50(1):3-32.
5 Kuzminski AM, Del Giacco EJ, Allen RH, et al: Effective Treatment of Cobalamin Deficiency with Oral Cobalamin. Blood 1998; 92:1191-1198.
6 Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, and Ulrich CM: Unmetabolized Folic Acid in Plasma is Associated with Reduced Natural Killer Cell Cytotoxicity among Postmenopausal Women. Journal of Nutrition 2006 Jan; 136(1): 189-194.
Rev. 10/19