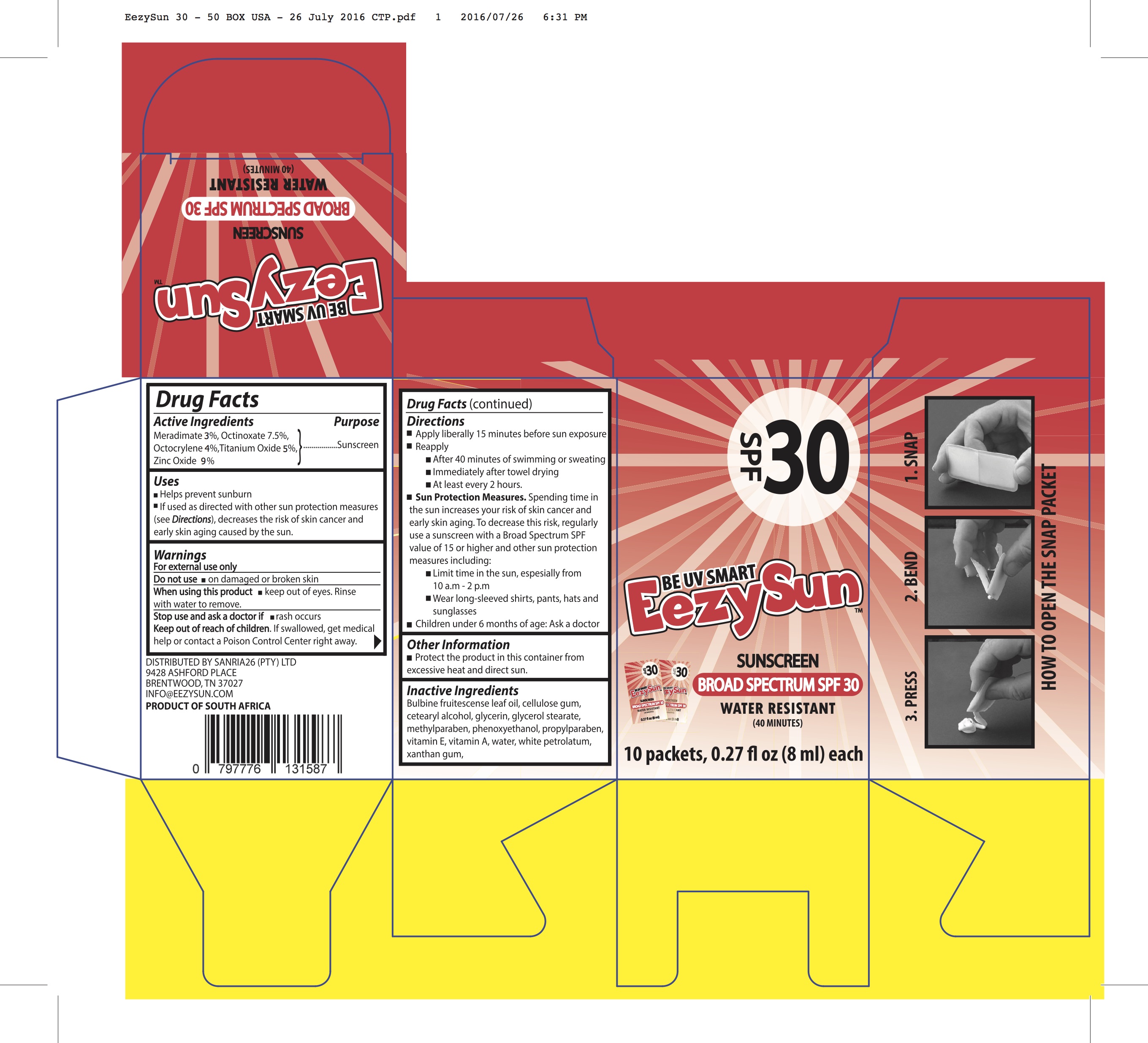
Inactives
Bulbine Fruitescence leaf oil, Cellulose gum, Cetearyl alcohol, Glycerin, Glycerol Stearate, Methylparaben, Phenoxyethanol, Propylparaben, Vit E, Vit A, Water, White petrolatum, Xanthan gum
Dosage and Administration
Directions
Apply liberally 15 minutes before sun exposure Reapply
PLEASE RECYCLE
DISTRIBUTED BY SANRIA26 (PTY) LTD 9428 ASHFORD PLACE BRENTWOOD, TN 37027 INFO@EEZYSUN.COM PRODUCT OF SOUTH AFRICA
After 40 minutes of swimming or sweating Immediately after towel drying
At least every 2 hours.
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
Limit time in the sun, espesially from 10 a.m - 2 p.m
Wear long-sleeved shirts, pants, hats and sunglasses Children under 6 months of age: Ask a docto