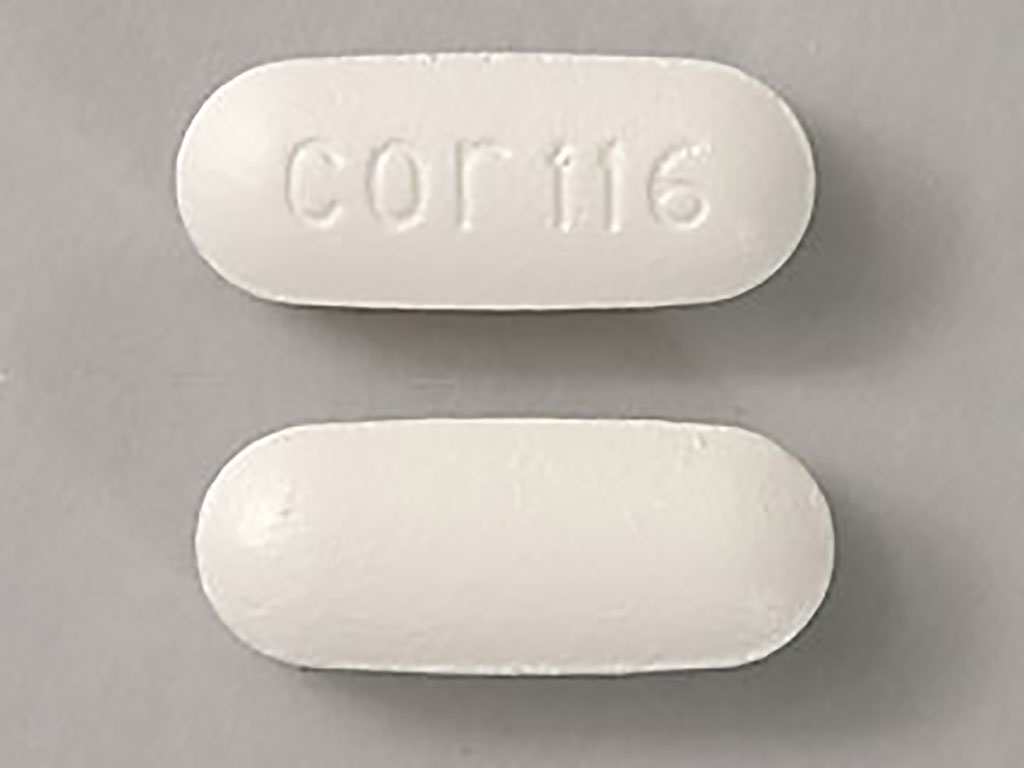
Uses
- temporarily relieves minor aches and pains due to:
- minor pain of arthritis
- muscular aches
- backache
- premenstrual and menstrual cramps
- the common cold
- headache
- toothache
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 6 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product.
Directions
- do not take more than directed (see overdose warning)
|
adults |
|
|
under 18 years of age |
|
Other information
- store at 20° to 25°C (68° to 77°F). Avoid excessive heat 40°C (104°F).
- FOR YOUR PROTECTION: Do not use if blister is torn or broken.
- supplied as unit dose packages of 30 (5 x 6) NDC 68084-777-25
Inactive ingredients
crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, povidone, pregelatinized starch, propylene glycol, sodium lauryl sulfate, stearic acid, titanium dioxide
Questions?
- about the drug product, call Sun Pharmaceutical Industries, Inc. at 1-800-406-7984
- about the packaging, call American Health Packaging at 1-800-707-4621
Contains No Aspirin
PACKAGING INFORMATION
American Health Packaging unit dose blisters contain drug product from Ohm Laboratories Inc. as follows:
(650 mg / 30 UD) NDC 68084-777-25 packaged from NDC 51660-333
Distributed by:
American Health Packaging
Columbus, OH 43217
8277725/0121
Principal Display Panel – Carton – 650 mg

NDC 68084- 777-25
Acetaminophen
Extended-release Tablets, USP
Pain Reliever/Fever Reducer
650 mg
30 Tablets (5 x 6)
DO NOT USE WITH OTHER MEDICINES
CONTAINING ACETAMINOPHEN.
Drug Facts
Active Ingredient Purpose
(in each caplet)
Acetaminophen USP, 650 mg ................. Pain reliever/fever reducer
Uses
See package insert for complete Drug Facts information.
Warnings
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if you take • more than 6 caplets
in 24 hours, which is the maximum daily amount • with other
drugs containing acetaminophen • 3 or more alcoholic drinks
every day while using this product
Allergy alert: acetaminophen may cause severe skin reactions.
Symptoms may include: • skin reddening • blisters • rash
If a skin reaction occurs, stop use and seek medical help right
away.
Keep out of reach of children.
See package insert for additional Drug Facts warnings.
Directions
• do not take more than directed, see package insert for
overdose warning and complete Drug Facts information.
Other Information • store at 20° to 25°C (68° to 77°F).
Avoid excessive heat 40°C (104°F). •
FOR YOUR
PROTECTION: Do not use product if blister is torn or broken.
Contains No Aspirin
The drug product contained in this package is
from NDC # 51660-333, Ohm Laboratories Inc.
Distributed by: American Health Packaging
2550 John Glenn Avenue, Suite A
Columbus, OH 43217
077725
0277725/0222