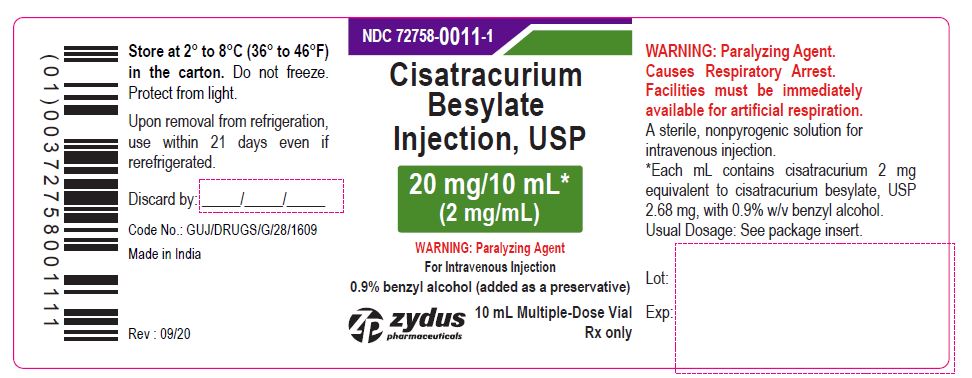
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 72758-0011-1
Cisatracurium Besylate Injection, USP
20 mg/10 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
0.9% benzyl alcohol (added as a preservative)
10 mL Multiple-Dose Vial
Rx only
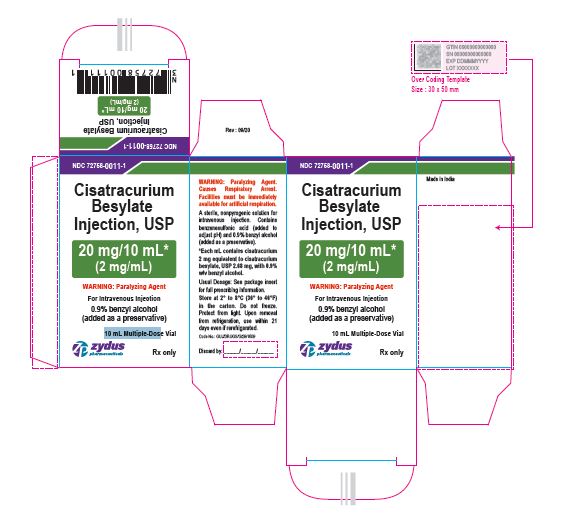
NDC 72758-0011-1
Cisatracurium Besylate Injection, USP
20 mg/10 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
0.9% benzyl alcohol (added as a preservative)
10 mL Multiple-Dose Vial
Rx only
NDC 72758-0011-6
Cisatracurium Besylate Injection, USP
20 mg/10 mL*
(2 mg/mL)
WARNING: Paralyzing Agent
For Intravenous Injection
0.9% benzyl alcohol (added as a preservative)
10 x 10 mL Multiple-Dose Vial
Rx only
