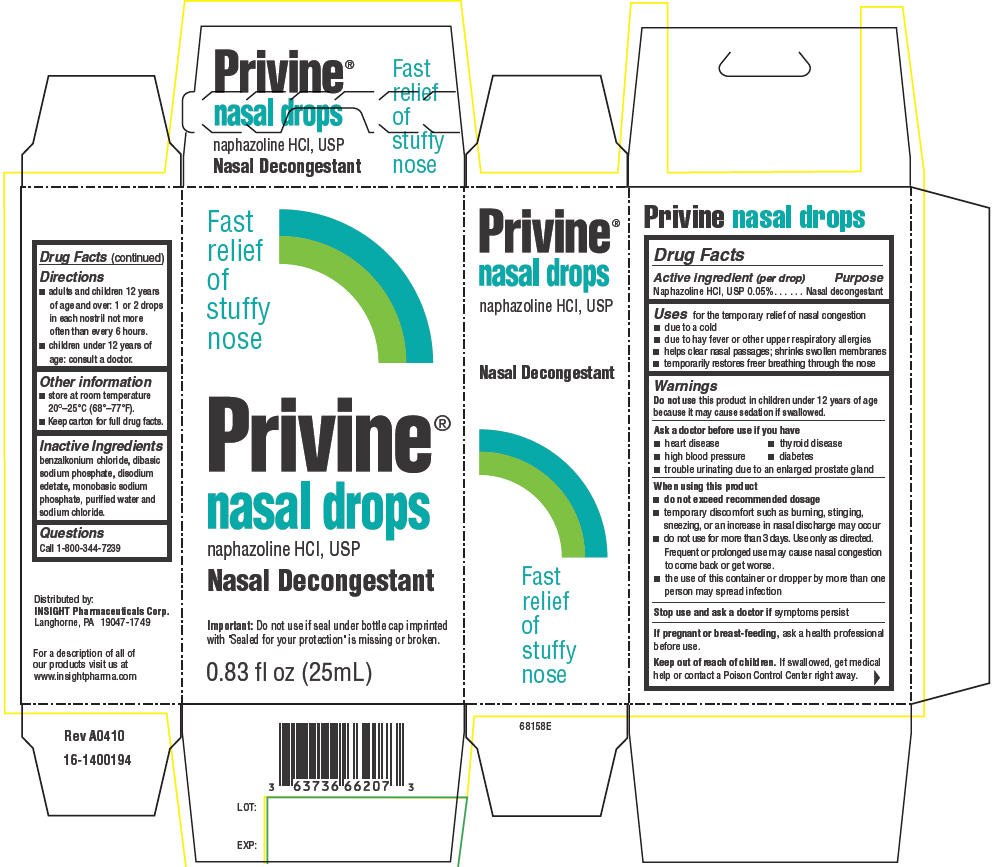
PRIVINE- naphazoline hydrochloride suspension/ drops
Insight Pharmaceuticals LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient (per drop)
Naphazoline HCl, USP 0.05%
Purpose
Nasal decongestant
Uses
for the temporary relief of nasal congestion
- due to a cold
- due to hay fever or other upper respiratory allergies
- helps clear nasal passages; shrinks swollen membranes
- temporarily restores freer breathing through the nose
Warnings
Do not use this product in children under 12 years of age because it may cause sedation if swallowed.
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
-
do not exceed recommended dosage
- temporary discomfort such as burning, stinging, sneezing, or an increase in nasal discharge may occur
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to come back or get worse.
- the use of this container or dropper by more than one person may spread infection
Stop use and ask a doctor if symptoms persist
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years of age and over: 1 or 2 drops in each nostril not more often than every 6 hours.
- children under 12 years of age: consult a doctor.
Other information
- store at room temperature 20°–25°C (68°–77°F).
- Keep carton for full drug facts.
Inactive Ingredients
benzalkonium chloride, dibasic sodium phosphate, disodium edetate, monobasic sodium phosphate, purified water and sodium chloride.
Questions
Call 1-800-344-7239
Distributed by:
INSIGHT Pharmaceuticals Corp.
Langhorne, PA 19047-1749
PRINCIPAL DISPLAY PANEL - 25 mL Drops Carton
Fast
relief
of
stuffy
nose
Privine®
nasal drops
naphazoline HCl, USP
Nasal Decongestant
Important: Do not use if seal under bottle cap imprinted
with "Sealed for your protection" is missing or broken.
0.83 fl oz (25mL)