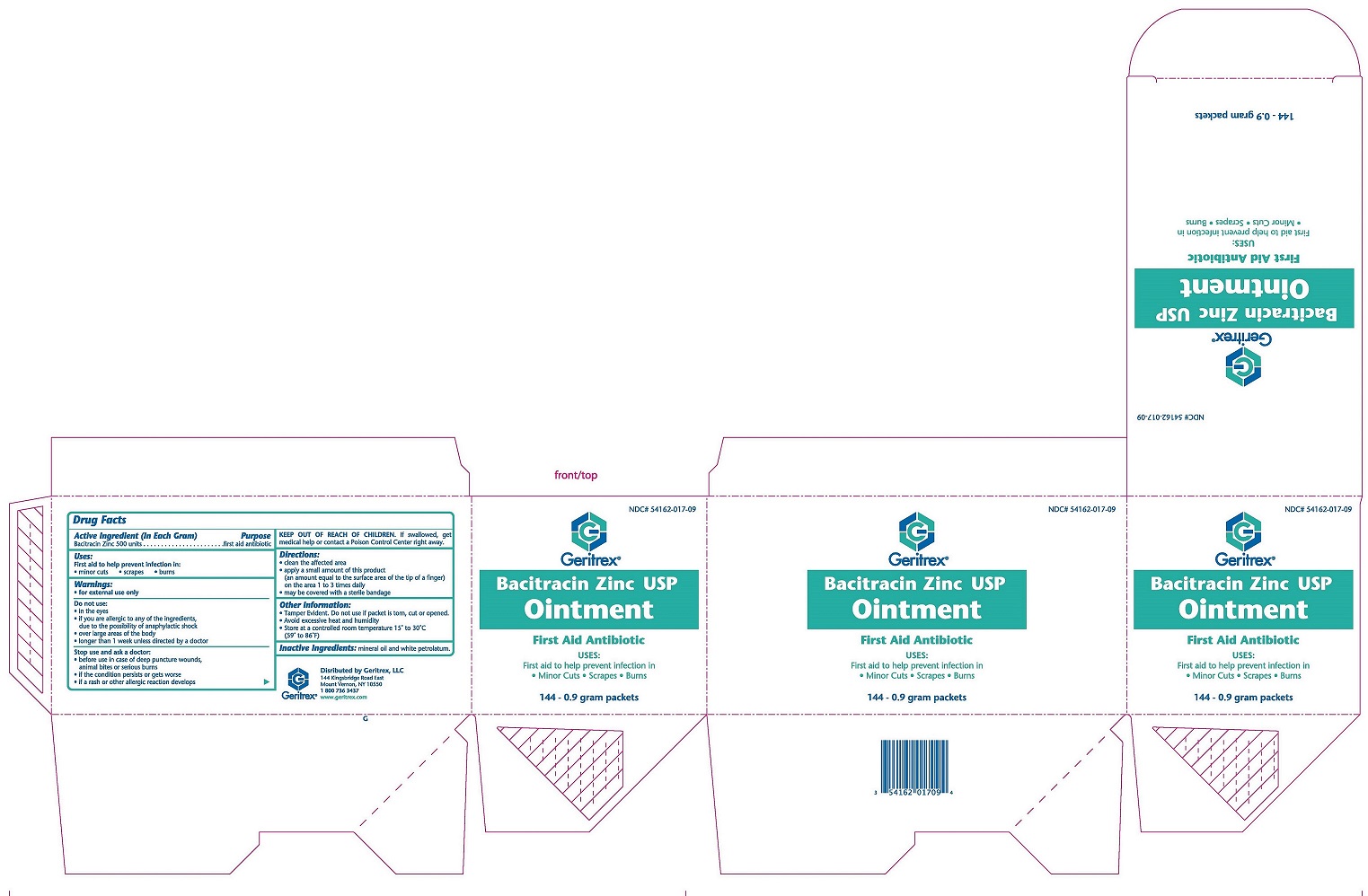
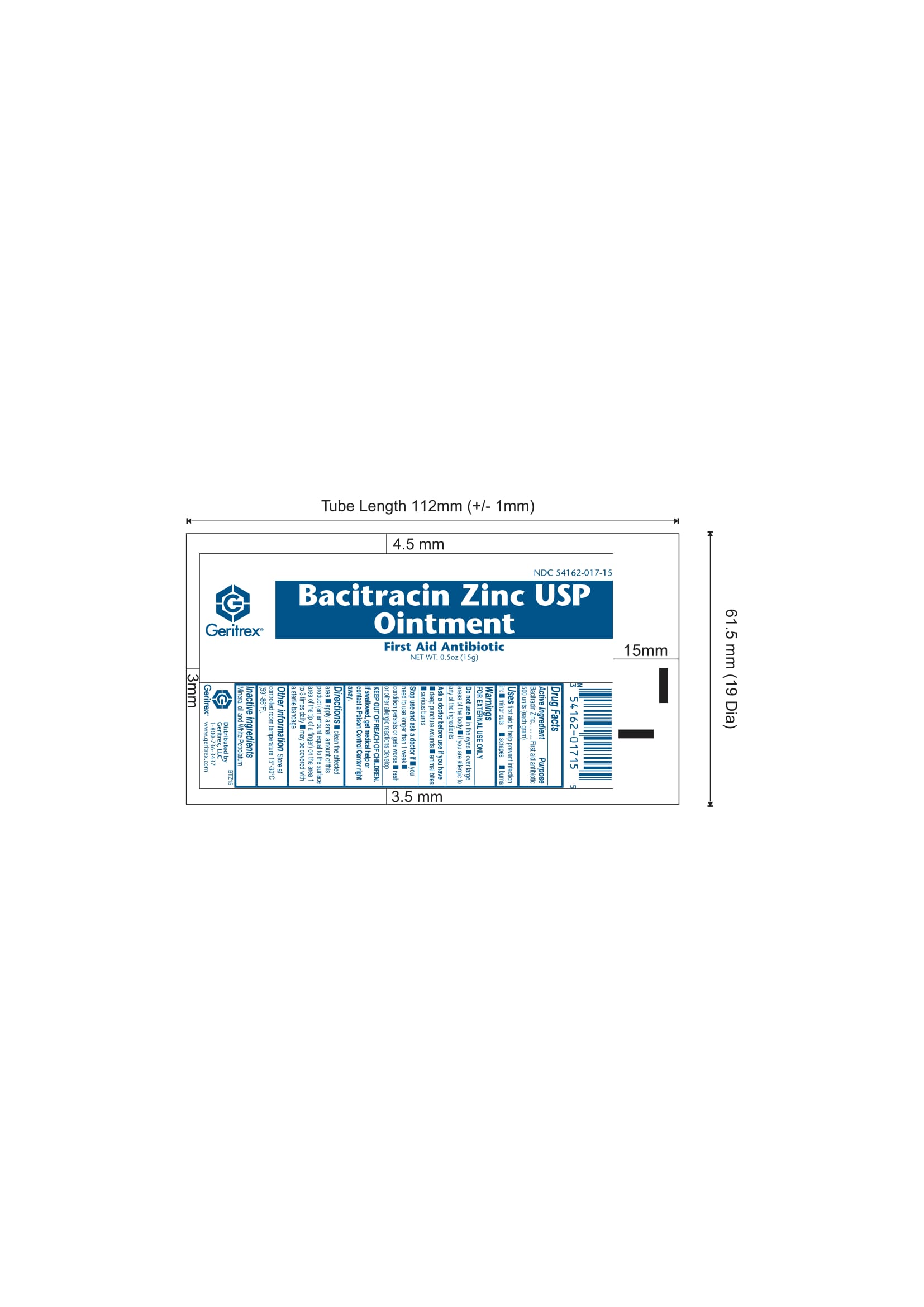
Active Ingredient: Purpose:
Bacitracin Zinc 500 units (each gram)....................First Aid Antibiotic
Warnings:
FOR EXTERNAL USE ONLY
Allergy Alert: Do not use if allergic to any of the ingredients.
Do not use in eyes or on large areas of the body.
Ask a doctor before use if you have deep or puncture wounds, animal bites or serious burns.
When using this product, do not use longer than 1 week, unless directed by a doctor.
Stop use and ask a doctor if condition lasts or gets worse, rahs or other allergic reaction develops.
KEEP OUT OF REACH OF CHILDREN
In the event of accidental ingestion, contact a Poison Control Center right away.