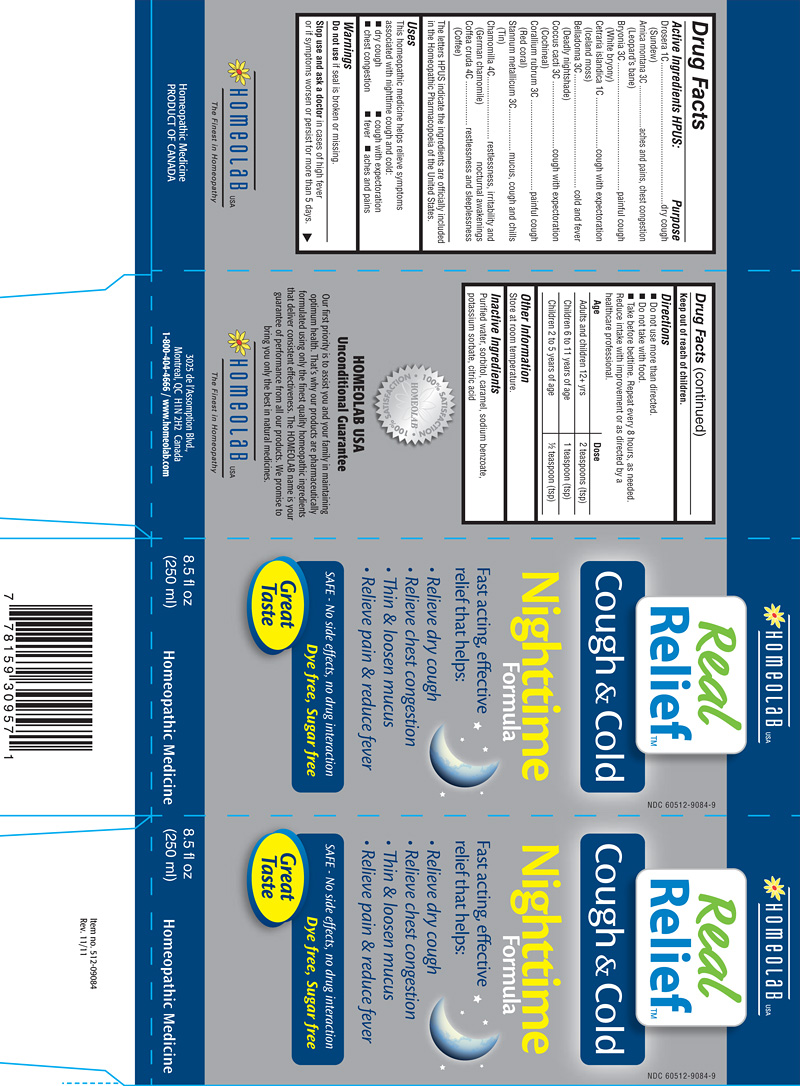
ACTIVE INGREDIENTS HPUS
Drosera (Sundew) 1C
Arnica montana (Leopard's bane) 3C
Bryonia (White bryony) 3C
Cetraria islandica (Iceland moss) 1C
Belladonna (Deadly nightshade) 3C
Coccus cacti (Cochineal) 3C
Corallium rubrum (Red coral) 3C
Stannum metallicum (Tin) 3C
Chamomilla (German chamomile) 4C
Coffea cruda (Coffee) 4C
PURPOSE
dry cough
aches and pains, chest congestion
painful cough
cough with expectoration
cold and fever
cough with expectoration
painful cough
mucus, cough and chills
restlessness, irritability and nocturnal awakenings
restlessness and sleeplessness
The letters HPUS indicate the ingredients are officially included in the Homeopathic Pharmacopoeia of the United States.
USES
This homeopathic medecine helps relieve symptoms associated with nighttime cough and cold:
- dry cough
- cough with expectoration
- chest congestion
- fever
- aches and pains
WARNINGS
DIRECTIONS
Do not use more than directed.
Do not take with food.
Take before bedtime. Repeat every 8 hours, as needed.
Reduce intake with improvement or as directed by a healthcare professional.
Adults and children 12+ yrs: 2 teaspoons.
Children 6 to 11 years of age: 1 teaspoon.
Children 2 to 5 years of age: 1/2 teaspoon.