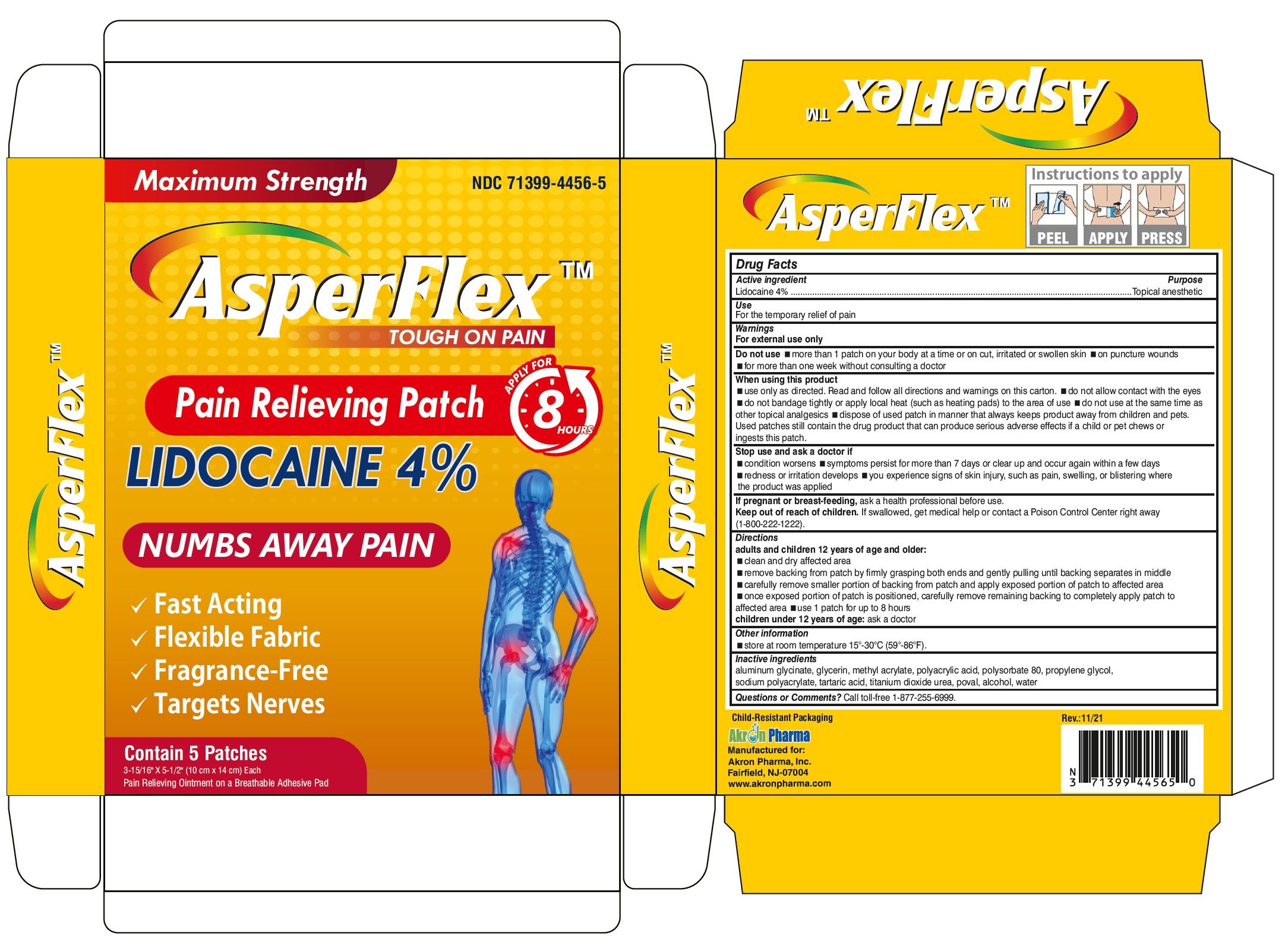
Warnings
For external use only
Do not use
- on puncture wounds, cuts, irritated or swollen skin
- more than 1 patch on your body at a time or with other topical analgesics at the same time
- with a heating pad or apply local heat to the area of use
When using this product
- use only as directed. Read and follow all directions and warnings on this carton.
- do not allow contact with the eyes
- do not bandage tightly or apply local heat (such as heating pads) to the area of use
- do not use at the same time as other topical analgesics
- dispose of used patch in manner that always keeps product away from children and pets. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this patch
Directions
adults and children over 12 years:
- clean and dry affected area
- remove backing from patch by firmly grasping both ends and gently pulling until backing separates in middle
- carefully remove smaller portion of backing from patch and apply exposed portion of patch to affected area
- once exposed portion of patch is positioned, carefully remove remaining backing to completely apply patch to affected area
- use 1 patch at a time and not more than 3 to 4 times daily
children 12 years or younger: consult a doctor
Inactive ingredients
aluminum glycinate, glycerin, methyl acrylate, polyacrylic acid, polysorbate 80, propylene glycol, sodium polyacrylate, tartaric acid, titanium dioxide urea, poval, alcohol, water