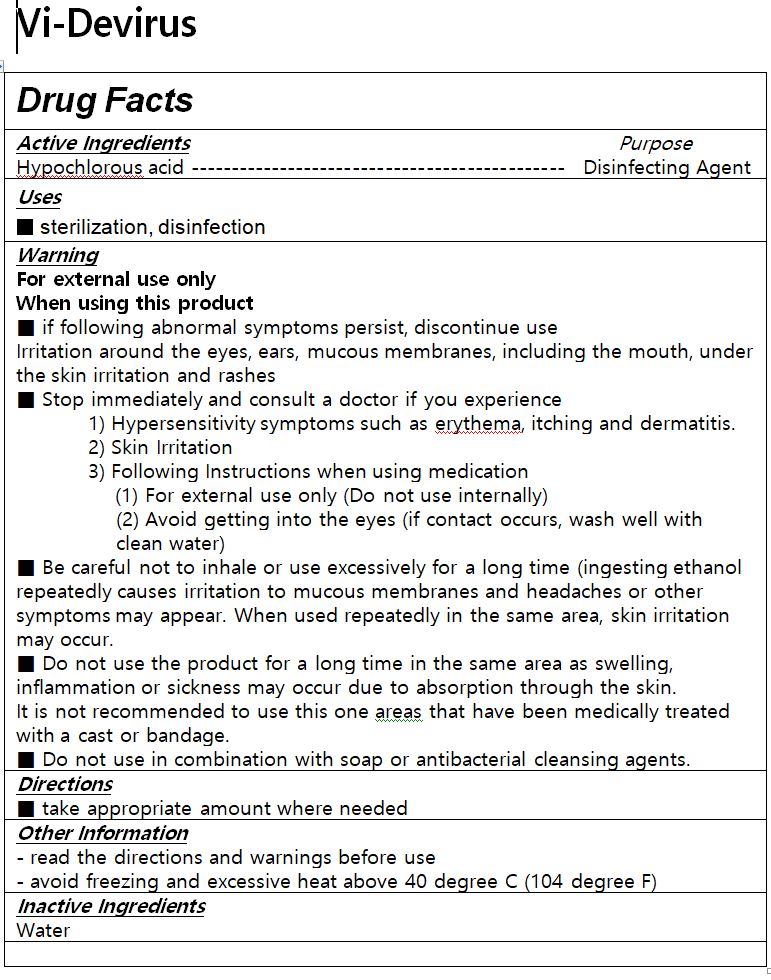
1. Do not use on the following body parts. A wide range of body parts and damaged skin around the eyes and ears, in the oral cavity (may have irritating effects)
2. If the following symptoms appear, stop using them immediately and consult a doctor or pharmacist.
1) Hypersensitivity symptoms such as rash, erythema, itching, and edema
2) Skin irritation symptoms