DESCRIPTION
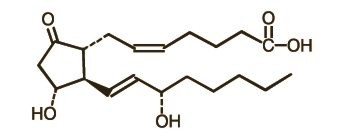
PREPIDIL Gel contains dinoprostone as the naturally occurring form of prostaglandin E2 (PGE2) and is designated chemically as (5Z, 11a, 13E, 15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid. The molecular formula is C20H32O5 and the molecular weight is 352.5. Dinoprostone occurs as a white to off-white crystalline powder with a melting point within the range of 65° to 69°C. It is soluble in ethanol, in 25% ethanol in water, and in water to the extent of 130 mg/100 mL. The active constituent of PREPIDIL Gel is dinoprostone 0.5 mg/3 g (2.5 mL gel); other constituents are colloidal silicon dioxide NF (240 mg/3 g) and triacetin USP (2760 mg/3 g).
The structural formula is represented below:
CLINICAL PHARMACOLOGY
PREPIDIL Gel (dinoprostone) administered endocervically may stimulate the myometrium of the gravid uterus to contract in a manner similar to contractions seen in the term uterus during labor. Whether or not this action results from a direct effect of dinoprostone on the myometrium has not been determined. Dinoprostone is also capable of stimulating smooth muscle of the gastrointestinal tract in humans. This activity may be responsible for the vomiting and/or diarrhea that is occasionally seen when dinoprostone is used for preinduction cervical ripening.
In laboratory animals, and also in humans, large doses of dinoprostone can lower blood pressure, probably as a result of its effect on smooth muscle of the vascular system. With the doses of dinoprostone used for cervical ripening this effect has not been seen. In laboratory animals, and also in humans, dinoprostone can elevate body temperature; however, with the dosing used for cervical ripening this effect has not been seen.
In addition to an oxytocic effect, there is evidence suggesting that this agent has a local cervical effect in initiating softening, effacement, and dilation. These changes, referred to as cervical ripening, occur spontaneously as the normal pregnancy progresses toward term and allow evacuation of uterine contents by decreasing cervical resistance at the same time that myometrial activity increases. While not completely understood, biochemical changes within the cervix during natural cervical ripening are similar to those following PGE2-induced ripening. Further, it has been shown that these changes can take place independent of myometrial activity; however, it is quite likely that PGE2 administered endocervically produces effacement and softening by combined contraction-inducing and cervical-ripening properties. There is evidence to suggest that the changes that take place within the cervix are due to collagen degradation resulting from collagenase secretion as a response, at least in part, to PGE2.
Using an unvalidated assay, the following information was determined. When PREPIDIL Gel was administered endocervically to women undergoing preinduction ripening, results from measurement of plasma levels of the metabolite 13,14-dihydro-15-keto-PGE2 (DHK-PGE2) showed that PGE2 was relatively rapidly absorbed and the Tmax was 0.5 to 0.75 hours. Plasma mean Cmax for gel-treated subjects was 433 ± 51 pg/mL versus 137 ± 24 pg/mL for untreated controls. In those subjects in which a clinical response was observed, mean Cmax was 484 ± 57 pg/mL versus 213 ± 69 pg/mL in nonresponders and 219 ± 92 pg/mL in control subjects who had positive clinical progression toward normal labor. These elevated levels in gel-treated subjects appear to be largely a result of absorption of PGE2 from the gel rather than from endogenous sources.
PGE2 is completely metabolized in humans. PGE2 is extensively metabolized in the lungs, and the resulting metabolites are further metabolized in the liver and kidney. The major route of elimination of the products of PGE2 metabolism is the kidneys.
INDICATIONS AND USAGE
PREPIDIL Gel is indicated for ripening an unfavorable cervix in pregnant women at or near term with a medical or obstetrical need for labor induction.
CONTRAINDICATIONS
Endocervically administered PREPIDIL Gel is not recommended for the following:
- a.
- Patients in whom oxytocic drugs are generally contraindicated or where prolonged contractions of the uterus are considered inappropriate, such as:
- cases with a history of cesarean section or major uterine surgery
- cases in which cephalopelvic disproportion is present
- cases in which there is a history of difficult labor and/or traumatic delivery
- grand multiparae with six or more previous term pregnancies cases with non-vertex presentation
- cases with hyperactive or hypertonic uterine patterns
- cases of fetal distress where delivery is not imminent
- in obstetric emergencies where the benefit-to-risk ratio for either the fetus or the mother favors surgical intervention
- b.
- Patients with hypersensitivity to prostaglandins or constituents of the gel (see WARNINGS and ADVERSE REACTIONS).
- c.
- Patients with placenta previa or unexplained vaginal bleeding during this pregnancy.
- d.
- Patients for whom vaginal delivery is not indicated, such as vasa previa or active herpes genitalia.
WARNINGS
FOR HOSPITAL USE ONLY
Dinoprostone, as with other potent oxytocic agents, should be used only with strict adherence to recommended dosages. Dinoprostone should be administered by physicians in a hospital that can provide immediate intensive care and acute surgical facilities.
Women aged 30 years or older, those with complications during pregnancy and those with a gestational age over 40 weeks have been shown to have an increased risk of post-partum disseminated intravascular coagulation. In addition, these factors may further increase the risk associated with labor induction (see ADVERSE REACTIONS). Therefore, in these women, use of dinoprostone should be undertaken with caution. Measures should be applied to detect as soon as possible an evolving fibrinolysis in the immediate post-partum phase.
The Clinician should be alert that the intracervical placement of dinoprostone gel may result in inadvertent disruption and subsequent embolization of antigenic tissue causing in rare circumstances the development of Anaphylactoid Syndrome of Pregnancy (Amniotic Fluid Embolism).
There have been post-marketing reports of serious and life-threatening hypersensitivity reactions including anaphylaxis and angioedema with PREPIDIL Gel (dinoprostone). Onset of these reported reactions occurred within minutes to hours after initiation with PREPIDIL Gel (dinoprostone). If a hypersensitivity reaction is suspected, if possible remove PREPIDIL Gel (dinoprostone). Assess for other potential causes of the event, and institute symptomatic and supportive therapy, as needed.
PRECAUTIONS
1. General Precautions
During use, uterine activity, fetal status, and character of the cervix (dilation and effacement) should be carefully monitored either by auscultation or electronic fetal monitoring to detect possible evidence of undesired responses, e.g., hypertonus, sustained uterine contractility, or fetal distress. In cases where there is a history of hypertonic uterine contractility or tetanic uterine contractions, it is recommended that uterine activity and the state of the fetus should be continuously monitored. The possibility of uterine rupture should be borne in mind when high-tone myometrial contractions are sustained. Feto-pelvic relationships should be carefully evaluated before use of PREPIDIL Gel (see CONTRAINDICATIONS).
Caution should be exercised in administration of PREPIDIL Gel in patients with:
- asthma or history of asthma
- glaucoma or raised intraocular pressure
Caution should be taken so as not to administer PREPIDIL Gel above the level of the internal os. Careful vaginal examination will reveal the degree of effacement which will regulate the size of the shielded endocervical catheter to be used. That is, the 20 mm endocervical catheter should be used if no effacement is present, and the 10 mm catheter should be used if the cervix is 50% effaced. Placement of PREPIDIL Gel into the extra-amniotic space has been associated with uterine hyperstimulation.
As PREPIDIL Gel is extensively metabolized in the lung, liver, and kidney, and the major route of elimination is the kidney, PREPIDIL Gel should be used with caution in patients with renal and hepatic dysfunction.
2. Patients With Ruptured Membranes
Caution should be exercised in the administration of PREPIDIL Gel in patients with ruptured membranes. The safety of use of PREPIDIL Gel in these patients has not been determined.
3. Drug Interactions
PREPIDIL Gel may augment the activity of other oxytocic agents and their concomitant use is not recommended. For the sequential use of oxytocin following PREPIDIL Gel administration, a dosing interval of 6–12 hours is recommended.
4. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenic bioassay studies have not been conducted in animals with PREPIDIL Gel due to the limited indications for use and short duration of administration. No evidence of mutagenicity was observed in the Micronucleus Test or Ames Assay.
5. Pregnancy,
Teratogenic Effects
Prostaglandin E2 produced an increase in skeletal anomalies in rats and rabbits. No effect would be expected clinically, when used as indicated, since PREPIDIL Gel is administered after the period of organogenesis. PREPIDIL Gel has been shown to be embryotoxic in rats and rabbits, and any dose that produces sustained increased uterine tone could put the embryo or fetus at risk. See statements under General Precautions.
ADVERSE REACTIONS
PREPIDIL Gel is generally well-tolerated. In controlled trials, in which 1731 women were entered, the following events were reported at an occurrence of ≥ 1%:
| Adverse Reaction | PGE2
(N = 884) | Control*
(N = 847) |
|---|---|---|
|
||
| Maternal | N (%) | N (%) |
| Uterine contractile abnormality | 58 (6.6) | 34 (4.0) |
| Any gastrointestinal effect | 50 (5.7) | 22 (2.6) |
| Back pain | 27 (3.1) | 0 (0) |
| Warm feeling in vagina | 13 (1.5) | 0 (0) |
| Fever | 12 (1.4) | 10 (1.2) |
| Fetal | ||
| Any fetal heart rate abnormality | 150 (17.0) | 123 (14.5) |
| Bradycardia | 36 (4.1) | 26 (3.1) |
| Deceleration | ||
| Late | 25 (2.8) | 18 (2.1) |
| Variable | 38 (4.3) | 29 (3.4) |
| Unspecified | 19 (2.1) | 19 (2.2) |
In addition, in other trials amnionitis and intrauterine fetal sepsis have been associated with extra-amniotic intrauterine administration of PGE2. Uterine rupture has been reported in association with the use of PREPIDIL Gel intracervically. Additional events reported in the literature, associated by the authors with the use of PREPIDIL Gel, included premature rupture of membranes, fetal depression (1 min Apgar < 7), and fetal acidosis (umbilical artery pH < 7.15).
Post-marketing surveillance
Blood and lymphatic system disorders
An increased risk of post-partum disseminated intravascular coagulation has been described in patients whose labor was induced by pharmacological means, either with dinoprostone or oxytocin (see WARNINGS). The frequency of this adverse event, however, appears to be rare (<1 per 1,000 labors).
DRUG ABUSE AND DEPENDENCE
No drug abuse or drug dependence has been seen with the use of PREPIDIL Gel.
OVERDOSAGE
Overdosage with PREPIDIL Gel may be expressed by uterine hypercontractility and uterine hypertonus. Because of the transient nature of PGE2-induced myometrial hyperstimulation, nonspecific, conservative management was found to be effective in the vast majority of the cases; i.e., maternal position change and administration of oxygen to the mother. β-adrenergic drugs may be used as a treatment of hyperstimulation following the administration of PGE2 for cervical ripening.
DOSAGE AND ADMINISTRATION
NOTE: USE CAUTION IN HANDLING THIS PRODUCT TO PREVENT CONTACT WITH SKIN. WASH HANDS THOROUGHLY WITH SOAP AND WATER AFTER ADMINISTRATION.
PREPIDIL Gel should be brought to room temperature (59° to 86°F; 15° to 30°C) just prior to administration. Do not force the warming process by using a water bath or other source of external heat (eg, microwave oven).
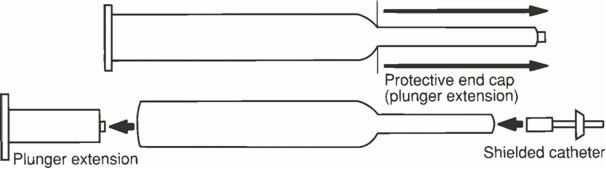
To prepare the product for use remove the protective end cap (to serve as plunger extension) and insert the protective end cap into the plunger stopper assembly in the barrel of syringe. Choose the appropriate length shielded catheter (10 mm or 20 mm) and aseptically remove the sterile shielded catheter from the package. Careful vaginal examination will reveal the degree of effacement which will regulate the size of the shielded endocervical catheter to be used. That is, the 20 mm endocervical catheter should be used if no effacement is present, and the 10 mm catheter should be used if the cervix is 50% effaced. Firmly attach the catheter hub to the syringe tip as evidenced by a distinct click. Fill the catheter with sterile gel by pushing the plunger assembly to expel air from the catheter prior to administration to the patient. Proper assembly of the dosing apparatus is shown below.
To properly administer the product, the patient should be in a dorsal position with the cervix visualized using a speculum. Using sterile technique, introduce the gel with the catheter provided into the cervical canal just below the level of the internal os. Administer the contents of the syringe by gentle expulsion and then remove the catheter. The gel is easily extrudable from the syringe. Use the contents of one syringe for one patient only. No attempt should be made to administer the small amount of gel remaining in the catheter. The syringe, catheter, and any unused package contents should be discarded after use. Following administration of PREPIDIL Gel, the patient should remain in the supine position for at least 15–30 minutes to minimize leakage from the cervical canal. If the desired response is obtained from PREPIDIL Gel, the recommended interval before giving intravenous oxytocin is 6–12 hours. If there is no cervical/uterine response to the initial dose of PREPIDIL Gel, repeat dosing may be given. The recommended repeat dose is 0.5 mg dinoprostone with a dosing interval of 6 hours. The need for additional dosing and the interval must be determined by the attending physician based on the course of clinical events. The maximum recommended cumulative dose for a 24-hour period is 1.5 mg of dinoprostone (7.5 mL PREPIDIL Gel).
HOW SUPPLIED
PREPIDIL Gel is available as a sterile semitranslucent viscous preparation for endocervical application: 0.5 mg PGE2 per 3.0 g (2.5 mL) in syringe. In addition, each package contains two shielded catheters (10 mm and 20 mm tip) enclosed in sterile envelopes. The contents are not guaranteed sterile if envelopes are not intact.
Each 3 gram syringe applicator contains:
dinoprostone, 0.5 mg; colloidal silicon dioxide, 240 mg; triacetin, 2760 mg.
| 1 × 3 gram syringe | NDC 0009-3359-01 |
| 5 × 3 gram syringes | NDC 0009-3359-02 |
This product's labeling may have been updated. For current full prescribing information, please visit www.pfizer.com
Rx only

LAB-0062-8.0
Revised 04/2019
PRINCIPAL DISPLAY PANEL - 3 g Syringe Applicator Label
NDC 0009-3359-01
Prepidil® Gel 0.5 mg*
dinoprostone cervical gel
For endocervical administration
Sterile
DOSAGE AND USE: See accompanying prescribing
information.
* Each 3 gram syringe applicator contains 0.5 mg
dinoprostone. Also contains 240 mg colloidal silicon
dioxide NF, 2760 mg triacetin USP.
Store in a refrigerator 2° to 8°C (36° to 46°F).
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc,
NY, NY 10017
LOT/EXP.:
Rx only
PAA056623

PRINCIPAL DISPLAY PANEL - 3 g Syringe Label
Prepidil® Gel 0.5 mg
dinoprostone cervical gel
Sterile
Store in a refrigerator 2° to 8°C (36° to 46°F).
One 3 gram syringe
Pfizer Injectables
Distributed by
Pharmacia & Upjohn Co
Division of Pfizer Inc, NY, NY 10017
NDC 0009-3359-01
PAA056625