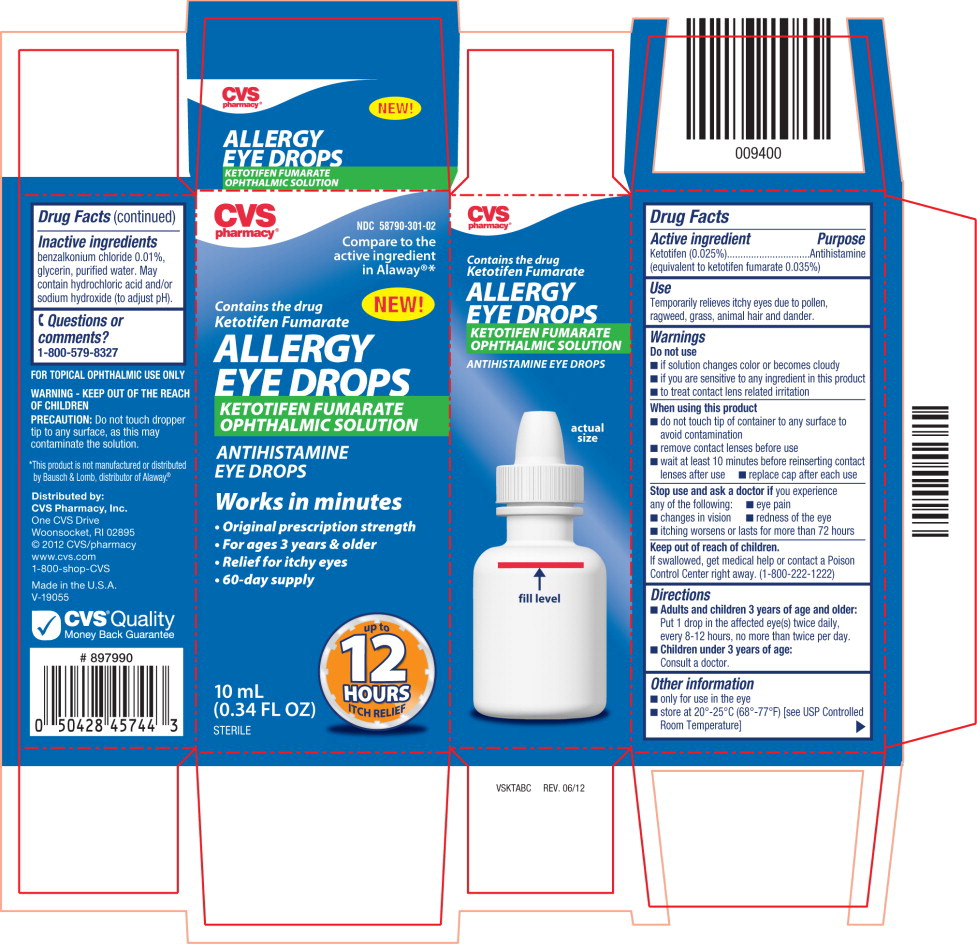
ALLERGY EYE DROPS- ketotifen fumarate solution/ drops
Advanced Vision Research (Subsidiary of Akorn, Inc.)
----------
Warnings
Do not use
- if solution changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
- to treat contact lens related irritation
Directions
-
Adults and children 3 years of age and older:
Put 1 drop in the affected eye(s) twice daily, every 8-12 hours, no more than twice per day.
-
Children under 3 years of age:
Consult a doctor.
Other information
- only for use in the eye
- store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature]
Inactive ingredients
benzalkonium chloride 0.01%, glycerin, purified water. May contain hydrochloric acid and/or sodium hydroxide (to adjust pH).
Principal Display Panel Text for Container Label:
CVS
pharmacy®
NDC 58790-301-02
ALLERGY
EYE DROPS
KETOTIFEN FUMARATE
OPHTHALMIC SOLUTION
ANTIHISTAMINE
EYE DROPS
10 mL (0.34 FL OZ) STERILE
Principal Display Panel Text for Carton Label:
CVS
pharmacy®
NDC 58790-301-02
Compare to the
active ingredient
in Alaway®*
NEW!
Contains the drug
Ketotifen Fumarate
ALLERGY
EYE DROPS
KETOTIFEN FUMARATE
OPHTHALMIC SOLUTION
ANTIHISTAMINE
EYE DROPS
Works in minutes
- Original prescription strength
- For ages 3 years & older
- Relief for itchy eyes
- 60-day supply
up to
12
HOURS
10 mL ITCH RELIEF
(0.34 FL OZ)
STERILE
| ALLERGY EYE DROPS
ketotifen fumarate solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Advanced Vision Research (Subsidiary of Akorn, Inc.) (117696736) |