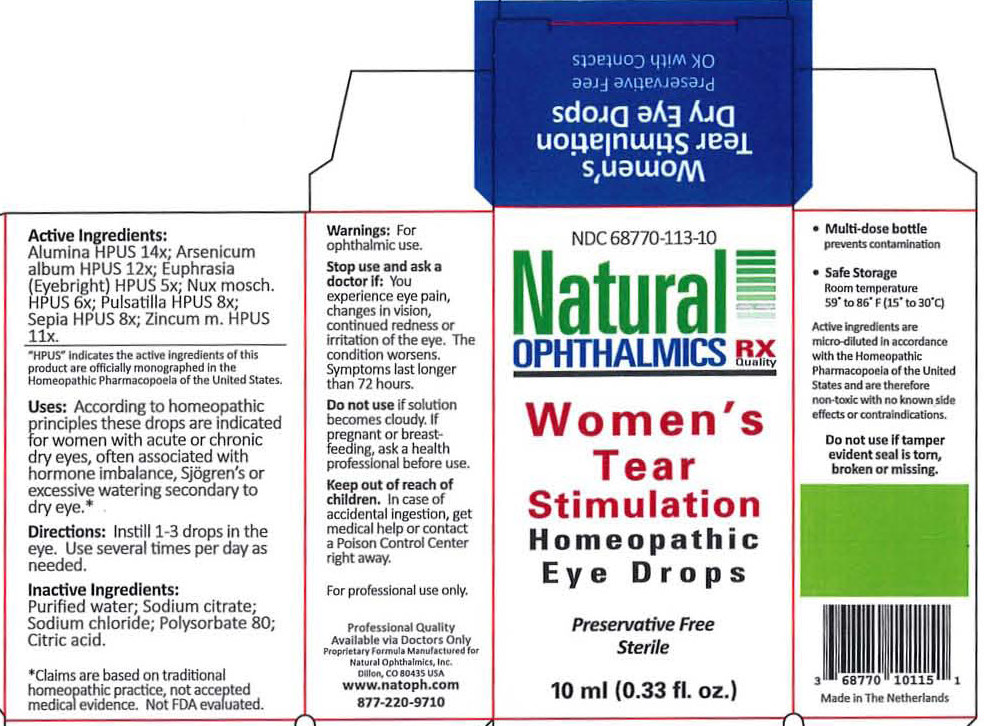
WOMENS TEAR STIMULATION- pulsatilla, sepia, euphrasia, alumina, arsenicum album, nux mosch, zincum m liquid
Natural Ophthalmics, Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Homeopathic Purpose
- Pulsatilla 8x }
- Sepia 8x }
- Euphrasia (Eyebright) 5x }
- Alumina 10x } Dry eyes
- Arsenicum album 12x }
- Nux mosch 6x }
- Zincum met 10x }
Active Ingredients
Pulsatilla 8x
Sepia 8x
Euphrasia 5x
Alumina 10x
Arsenicum album 12x
Nux mosch 6x
Zincum m 11x
Inactive Ingredients
Purified water
Polysorbate 80
Sodium citrate
Sodium chloride
Uses
According to homeopathic principles these drops are indicated for Women with acute or chronic dry eyes, often associated with hormone imbalance, Sjogren's or excessive watering secondary to dry eye.
Directions
Instill 1-3 drops in the eye. Use several times per day as needed.
Warnings
For ophthalmic use.
For Professional Use Only
Keep out of reach of children. Incase of accidental ingestion, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if
Stop use and ask a doctor if: You experience eye pain, changes in vision, continued redness or irritation of the eye. The condition worsens. Symptoms last longer than 72 hours.
Do not use
Do not use if solution becomes cloudy.
If pregnant or breast feeding
If pregnant or breast feeding, ask a health professional before use.
Storage
Room temperature
59
o to 86
o (15
o to 30
o C)
Natural Ophthalmics, Inc.
Lafayette, CO 80026 USA
www.natoph.com
877-220-9710
Label image