HEALTH CLAIM
XYZMUNE Capsules - Dietary Supplement
Dispensed by Prescription‡
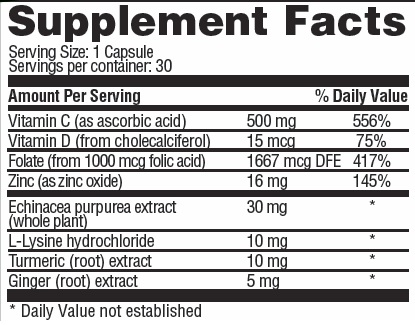
OTHER INGREDIENTS: gelatin, calcium stearate, silica, organic mushroom (Pleurotus eryngii), organic turkey tail (Trametes versicolor), organic cordyceps (Cordyceps militaris), organic reishi (Ganoderna lucidum) organic himematsutake (Agaricus blazei), organic lion's mane (Hericium erincaces), organic antrodia (Taiwanofungus camphoratus (Antrodia camphorate)), organic maitake (Grifola frondosa)
DESCRIPTION:
XYZMUNE is an orally administered prescription vitamin formulation for the clinical dietary management of suboptimal nutritional status in patients where advanced supplementation is required and nutritional supplementation in physiologically stressful conditions for maintenance of good immune health is needed.†
XYZMUNE is manufactured in accordance with Current Good Manufacturing Practice for foods, using ingredients that have been approved by the U.S. Food and Drug Administration (FDA) as food additives or are “Generally Recognized as Safe” (GRAS) for their intended use.
‡ XYZMUNE should be administered under the supervision of a licensed medical practitioner.
† These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure or prevent disease.
WARNING AND PRECAUTIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients. XYZMUNE capsules should only be used under the direction and supervision of a licensed medical practitioner.‡ Use with caution in patients that may have a medical condition, are pregnant, lactating, trying to conceive, under the age of 18, or taking medications.
KEEP OUT OF REACH OF CHILDREN
PRECAUTION
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamin B is deficient. Folic acid in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. XYZMUNE capsules should only be used under the direction and supervision of a licensed medical practitioner.
ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid. You may report side effects by calling 1-844-551-9911 or the FDA by calling 1-800-FDA-1088.
DOSAGE & ADMINISTRATION
Usual adult dose is 1 capsule taken orally once or twice daily or as prescribed by a licensed medical practitioner.
HOW SUPPLIED HEALTH CLAIM:
XYZMUNE Dietary Supplement
Capsules are clear with a yellow powder inside
Bottles contain 30 capsules, 69597-355-30*
Dispensed by Prescription‡
Manufactured in USA for:
Basiem, LLC
Madisonville, LA 70447
Distributed by: Solubiomix, LLC
Rev. 04/2020
* Basiem does not represent these product codes to be National Drug Codes (NDC). Product codes are formatted according to standard industry practice, to meet the formatting requirement by pedigree reporting and supply-chain control including pharmacies.
‡ The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product by prescription. This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable. The ingredients, indication or claims of this product are not to be construed to be drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR 20750)
2. Federal Register Notice of October 17, 1980 (45 FR 69043, 69044)
3. Federal Register Notice of March 5, 1996 (61 FR 8760)
