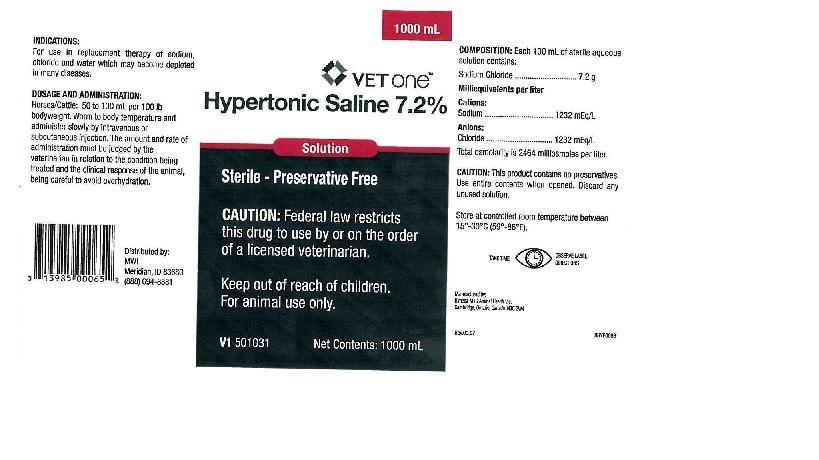
INDICATIONS:
For use in replacement therapy of sodium, chloride and water which may become depleted in many diseases.
DOSAGE AND ADMINISTRATION:
Horses/Cattle: 50 to 100 mL per 100 lb bodyweight. Warm to body temperature and administer slowly by intravenous or subcutaneous injection. The amount and rate of administration must be judged by the veterinarian in relation to the condition being treated and the clinical response of the animal, being careful to avoid overhydration.
COMPOSITION: Each 100 mL of sterile aqueous solution contains:
Sodium Chloride.............................7.2 g
Milliequivalents per liter
Cations:
Sodium.........................................1232mEq/L
Anions:
Chloride........................................1232mEq/L
Total osmolarity is 2464 milliomoles per liter.